INTRODUCTION
A comprehensive review has concluded that progenitor cells, which are distributed along the dorsal-ventral axis of the spinal cord in vertebrates, generate distinct types of neuron in each compartment (Briscoe & Ericson, 2001). Our previous study suggested that zebrafish Jagged2 interacts with progenitor cells in the adjacent p3 domain and plays a crucial role in maintaining proliferating neural progenitor cells in this region (Yeo & Chitnis, 2007). It was proposed that Jagged2 might bind to Notch expressed in the neural progenitor cells of the p3 domain, thereby facilitating signal transduction in a contact-dependent manner (Yeo & Chitnis, 2007). While the p3 domain in zebrafish is composed of a single cell layer, the pMN domain is composed of multiple cell layers (Yeo & Chitnis, 2007). Jagged2 is expressed in the pMN domain of the zebrafish, which encompasses neural precursors as well as various types of neurons (Yeo & Chitnis, 2007). Therefore, it can be concluded that Jagged2-mediated Notch signaling does not only occur between neural progenitors in the p3 domain and those in the pMN domain. This study investigates the activation and transmission mechanisms of Jagged2, which has previously been shown to activate the Notch signaling pathway.
The Notch signaling pathway can be classified into five different types based on three methods of classification: the number of cells involved, the type of cells involved, and the signaling distance. The first, canonical lateral inhibition, involves the short-range activation of the Notch signaling within equivalent adjacent cells (Chitnis, 1995). The second, long-range lateral inhibition, involves the transmission of intermittent Delta signals within equivalent cells by dynamic filopodia (Yatsenko & Shcherbata, 2021). Third, cis-inhibition involves Notch and Delta expressed on the same cell surface to suppress intrinsic Notch signal transmission (Sprinzak et al., 2010). Fourth, local induction involves the activation of Notch signaling within unequivalent cells that are adjacent to those receiving Jagged signaling (Yeo & Chitnis, 2007). Finally, long-distance induction is characterized by the activation of the Notch signaling pathway in non-equivalent cells that are distant from the Delta-sending cells (Yatsenko & Shcherbata, 2021).
We have investigated how Mib is involved in the regulation of Jagged2. The ubiquitination of Jagged2 by Mib has been shown to promote proteasome-dependent degradation in undifferentiated P19 cells. Co-immunoprecipitation (IP) analysis revealed that Mib physically interacts with Jagged2 but does not interact with the intracellular domain itself. In cell transplantation experiments, Jagged2:mRFP-positive cells formed extended filopodia and secreted extracellular vesicles (EVs). It was observed that co-expression of Mib:EGFP promotes the formation of EVs containing Jagged2. Based on these observations, it might be possible to determine which of the four aforementioned mechanisms the Mib-mediated Jagged2 signaling pathway corresponds to.
MATERIALS AND METHODS
Zebrafish were maintained as described by Yeo & Chitnis (2007). AB* wild-type were used.
Jagged2-related expression plasmids, pCS2MT-J2, pCS2MT-J2ICD, pCS2MT-J2ΔECD and pCS2MT-J2ΔICD were obtained from Ryu et al. (2024). Mib-related expression plasmids, pCS-HA-Mib, pCS-HA- pCS-HA-Mibta52b, pCS-HA-Mibtf101 and pCS-HA-Mibm178 were obtained from Itoh et al. (2003). pCS-HA-Ub were obtained from Itoh et al. (2003). The mRNAs were synthesized from the pCS-Mib:EGFP and pCS2MT-J2:mRFP constructs, and microinjection was performed as described by Yeo et al. (2001).
The P19 was grown in medium as described by Kong et al. (2018). COS cell line was grown in medium as described by Ryu et al. (2024). Co-IP was performed as described by Ryu et al. (2024). Proteins were separated by SDS-PAGE and detected by immunoblotting. Primary antibodies used were as follows: anti-Myc antibody (Sigma-Aldrich, St. Louis, MO, USA), anti-Flag M2 antibody (Sigma-Aldrich) and HA-tag antibody (Santa Cruz Biotechnology, Dallas, TX, USA). Secondary antibodies used were as describe by Yeo & Chitnis (2007).
All-trans retinoic acid (RA) was prepared as a stock solution at 10−2 M in 95% ethanol (Kong et al., 2018). RA was treated at P19 cells as described by Kong et al. (2018).
Cell transplantation was performed as described by Jung et al. (2012). Fluorescent images were taken at 7-hpf with a confocal laser scanning microscope (LSM 510; Carl Zeiss).
RESULTS
Previous studies have shown that the generation of GABAergic Kolmer-Agduhr neurons (KA) neurons in the p3 domain is regulated by Jagged2-mediated Notch signaling (Yeo & Chitnis, 2007). Furthermore, the generation of KA neurons has been found to be influenced by the overexpression of Mib (Ryu et al., 2015). First, we examined the ubiquitination of Jagged2 by the E3 ubiquitin ligase Mib in P19 cells, since the Notch-mediated lateral inhibition mechanism occurs in undifferentiated cells. The P19 cell line is a pluripotent embryonal teratocarcinoma, which differentiates into neuron and glial cells following RA treatment (Kong et al., 2018). Flag-tagged Mib and HA-tagged ubiquitin (HA-Ub) were co-transfected into P19 cells with the Myc-tagged full length Jagged2 (J2), the intracellular domain truncated forms (J2ΔICD), the extracellular domain truncated forms (J2ΔECD), or the intracellular domain (J2ICD). Immunoprecipiation (IP) with anti-Myc antibody and detection with anti-HA antibody showed that Mib enhances the ubiquitination of J2ΔICD, J2ΔECD and J2 (Lane 5, 8 and 11 in Fig. 1A), whereas that of J2ICD was undetectable in the presence of Mib in P19 cells (Lane 2 in Fig. 1A). Furthermore, the amount of ubiquitinated Jagged2 dramatically increased in the presence of MG132, which effectively blocked the proteolytic activity of the 26S proteasome complex (Lane 12 in Fig. 1A, Kong et al., 2018). This observation implicates that the ubiquitination of full-length Jagged2 by Mib promotes its proteasome-dependent degradation in undifferentiated P19 cells. Along with the quantitative changes in ubiquitinated Jagged2, changes in the amount of transfected proteins in the whole cell lysate were also observed. Western blot (WB) analysis showed that the presence of Mib in whole cell lysate resulted in a quantitative decrease in Jagged2 protein (asterisk in lane 11 in Fig. 1A). In contrast, the amount of Jagged2 and Mib dramatically increased in the presence of MG132 (asterisk and open asterisk in lane 12 in Fig. 1A). However, no quantitative change in Jagged2 variants was observed in the presence of MG132 (Lane 3, 6 and 9 in Fig. 1A). These observations suggest that Mib could promote ubiquitination of membrane-bound Jagged2 and its proteasome-dependent degradation in undifferentiated P19 cells.
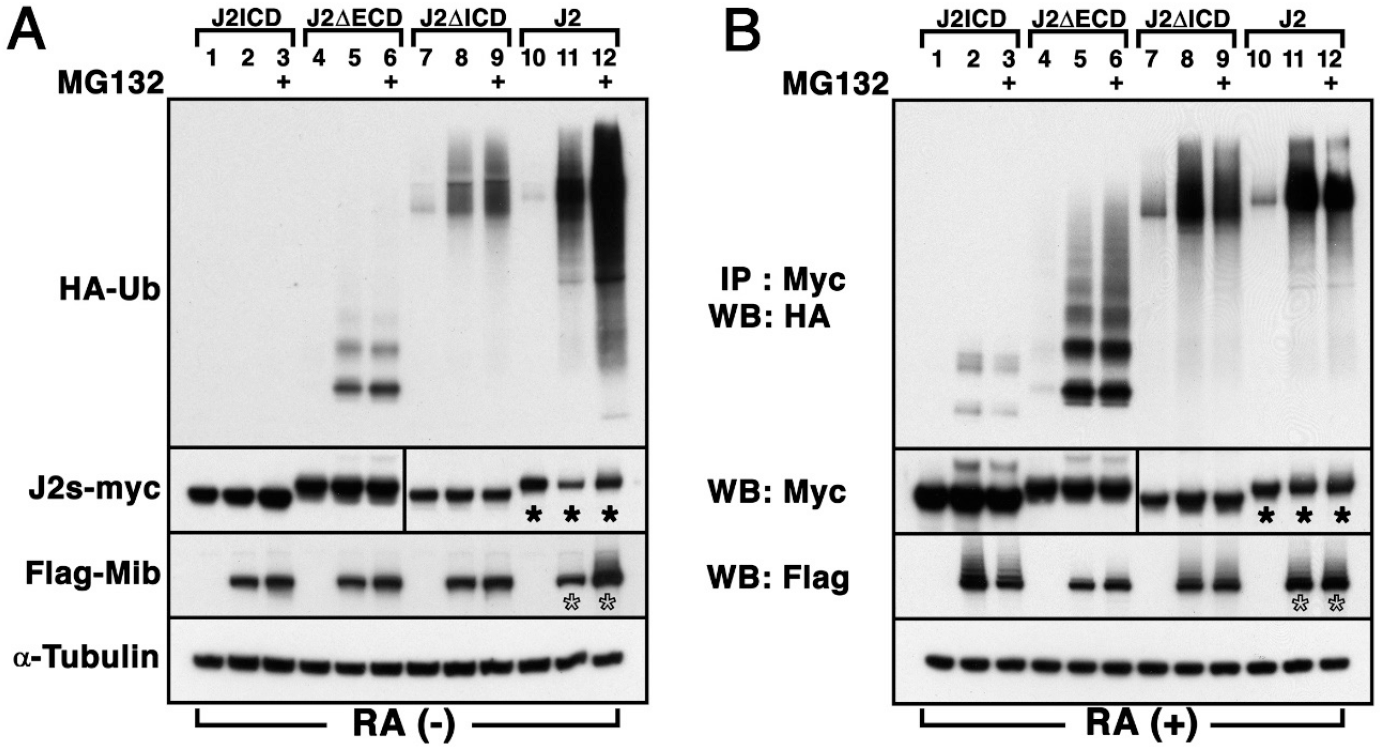
The lateral inhibition mechanism by the Notch signaling pathway occurs between undifferentiated and equivalent cells, meaning that one selected cell differentiates into a neuron during primary neurogenesis (Chitnis, 1995). Previous study showed that zebrafish Jagged2 was expressed in the pMN domain of spinal cord (Yeo & Chitnis, 2007). This pMN domain in the developing spinal cord contains at least three types of cells which are progenitors, the differentiating cells, and differentiated neurons (Yeo & Chitnis, 2007). Therefore, studying Jagged2-mediated Notch signaling by Mib in cells under various conditions is necessary. To assess the effect of ubiquitination on the function of Jagged2 in the differentiating neural progenitors, P19 cells were aggregated and grown in bacterial-grade Petri dishes in the presence of RA for a period of 4 days and subsequently plated on tissue culture dishes for co-transfection with various plasmids (Fig. 1B, Kong et al., 2018). Although the overall degree of ubiquitination of Jagged2 variants was slightly higher in RA-treated P19 cells than in P19 cells, we were unable to directly compare the degree of J2ΔECD ubiquitination in the absence of RA with that in its presence (Fig. 1B). Nevertheless, WB analysis clearly showed that the amount of Jagged2 remained unchanged in the presence of Mib in the whole cell lysate (asterisk of lane 11 in Figure 1B), whereas Mib enhanced the ubiquitination of J2ICD, J2ΔICD, J2ΔECD and J2 (lanes 2, 5, 8 and 11 in Fig. 1B, respectively). Furthermore, the levels of ubiquitinated Jagged2 and Mib also remained unchanged in the presence of MG132 in the whole cell lysate (asterisk and open asterisk of lane 12 in Fig. 1B). These observations suggest that Mib could also ubiquitinate Jagged2 even in RA-treated P19 cells, but does not promote its proteasome-dependent degradation in differentiating P19 cells. Furthermore, it was observed that Mib could ubiquitinate J2ΔICD, the truncated form of cytosolic domain of Jagged2. In addition, the ubiquitination of J2ICD, cytosolic domain of Jagged2, was either undetectable in undifferentiated P19 cells or detected at very low levels in differentiating P19 cells. These observations imply that Mib may function indirectly on the intracellular or extracellular domain of Jagged2 to ubiquitinate it, or that Mib may promote the overall ubiquitination levels in differentiating P19 cells.
To identify the requirements for interactions between Mib and Jagged2, we performed an IP assay by co-transfecting Flag-tagged Mib with either Myc-tagged Jagged2 (J2) or truncated forms of Jagged2 (Fig. 2A). Myc-tagged J2 and J2 ΔECD in the lysate was captured using anti-Myc antibody and precipitated along with Flag-tagged Mib using a resin in vitro. Co-IP analysis revealed that Mib was capable of interacting with J2ΔECD as well as J2, but not with J2ICD and J2ΔICD, in undifferentiated P19 cells (Fig. 2A). This observation support the idea from previous study that an adaptor such as PEX1, which can directly bind to J2ICD, might be necessary for interaction between Mib and Jagged2 (Ryu et al., 2024).
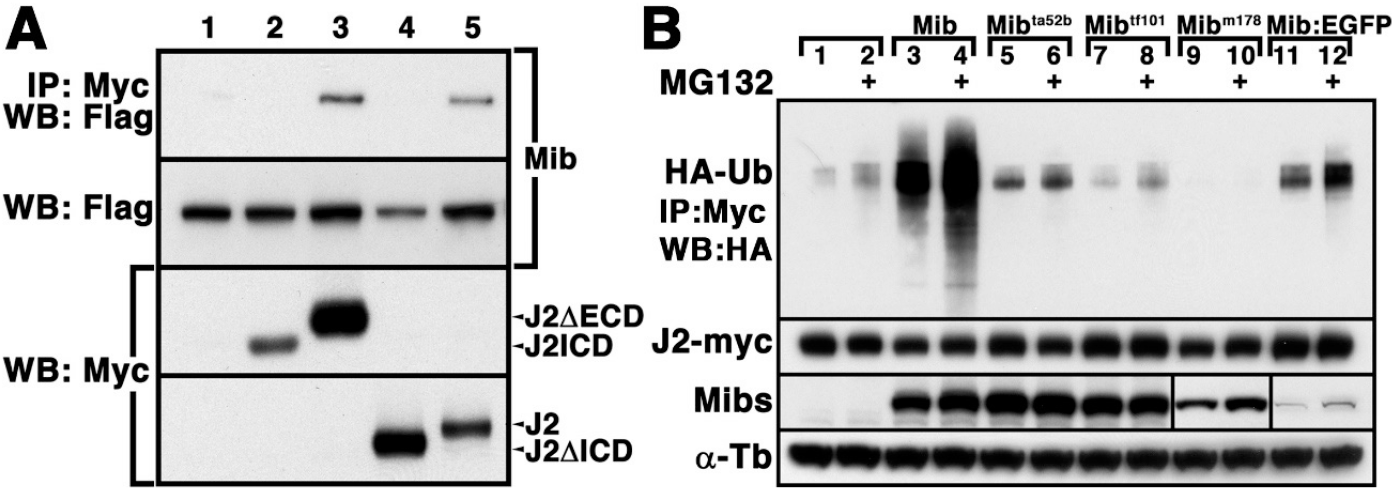
To successfully perform further experiments, such as the co-localization assay for Jagged2 and Mib, it was essential to evaluate the functionality of the EGFP-tagged Mib using a fluorescent assay. To compare the ubiquitination activity of wild-type Mib with that of EGFP-tagged Mib and mutant forms of Mib, Mibta52b, Mibtf101 and Mibm178 against Jagged2, Myc-tagged J2 was co-transfected with Mib variants into P19 cells (Fig. 2B). WB analysis showed that the ubiquitination of Jagged2 was slightly enhanced in the presence of Flag-Mibta52b and EGFP-tagged Mib but not by Mibtf101 and Mibm178 in P19 cells (Fig. 2B). In addition, ubiquitination of Jagged2 promoted its proteasome-dependent degradation in the presence of Mib, Mibta52b and EGFP-tagged Mib in P19 cells (Fig. 2B). These results suggested that Mib retained E3 ubiquitin ligase activity even when tagged with EGFP.
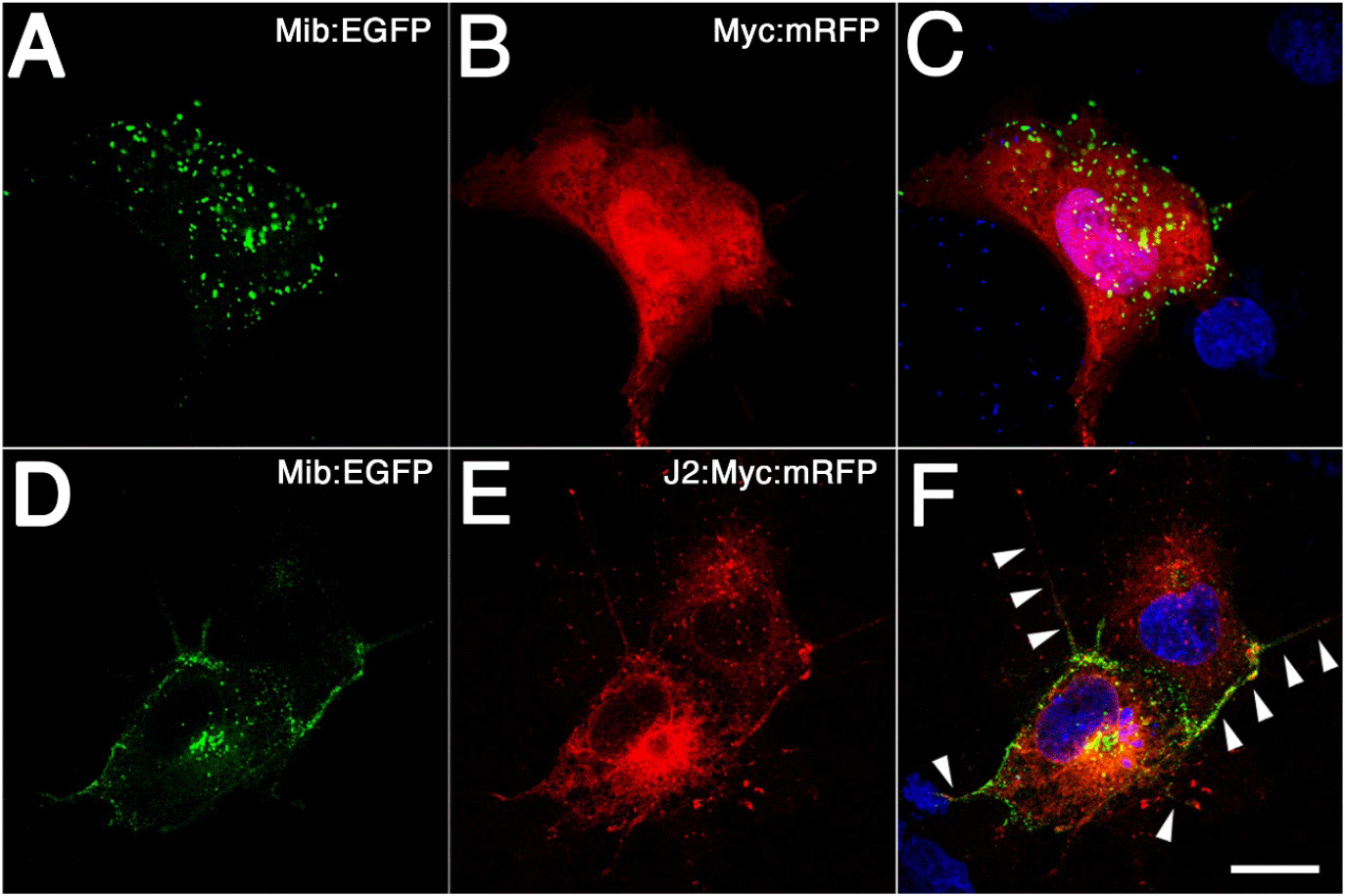
The physical interaction between Jagged2 and Mib was also tested in vivo by coexpressing these proteins in COS cells (Fig. 3). First, we confirmed the validity of this in vivo assay by coexpressing EGFP-tagged Mib in COS cells together with Myc-tagged mRFP (Fig. 3A and B). Confocal images showed that Mib was expressed in a dotted pattern, as in the HEK293 cell line (Fig. 3A, Yoo et al., 2006). Since Mib overlapped with Snx5 which was expressed in vesicular structures, these green dotted pattern could be seen as early endosomes (Fig. 3B, Yoo et al., 2006). Next, we performed immunofluorescence staining by coexpressing EGFP-tagged Mib in COS cells together with Myc and mRFP-tagged Jagged2 (Fig. 3D and E). Confocal images showed a change in the expression of Mib from a dotted pattern within the cytoplasm to one observed predominantly at the cell membrane (Fig. 3D). Jagged2 protein, which contains a transmembrane domain, was expected to be expressed primarily at the cell membrane. However, observations revealed that it was predominantly expressed in the cytoplasm, rather than exclusively at the membrane of COS cells (Fig. 3E). The merged images demonstrated that EGFP-tagged Mib partially overlapped with Myc-tagged Jagged2:mRFP in the cytoplasm; however, substantial overlap was observed in what was thought to be a filopodia (Fig. 3F). These changes in expression, as observed in a cultural setting, need to be confirmed in an in vivo system, such as a zebrafish embryo.
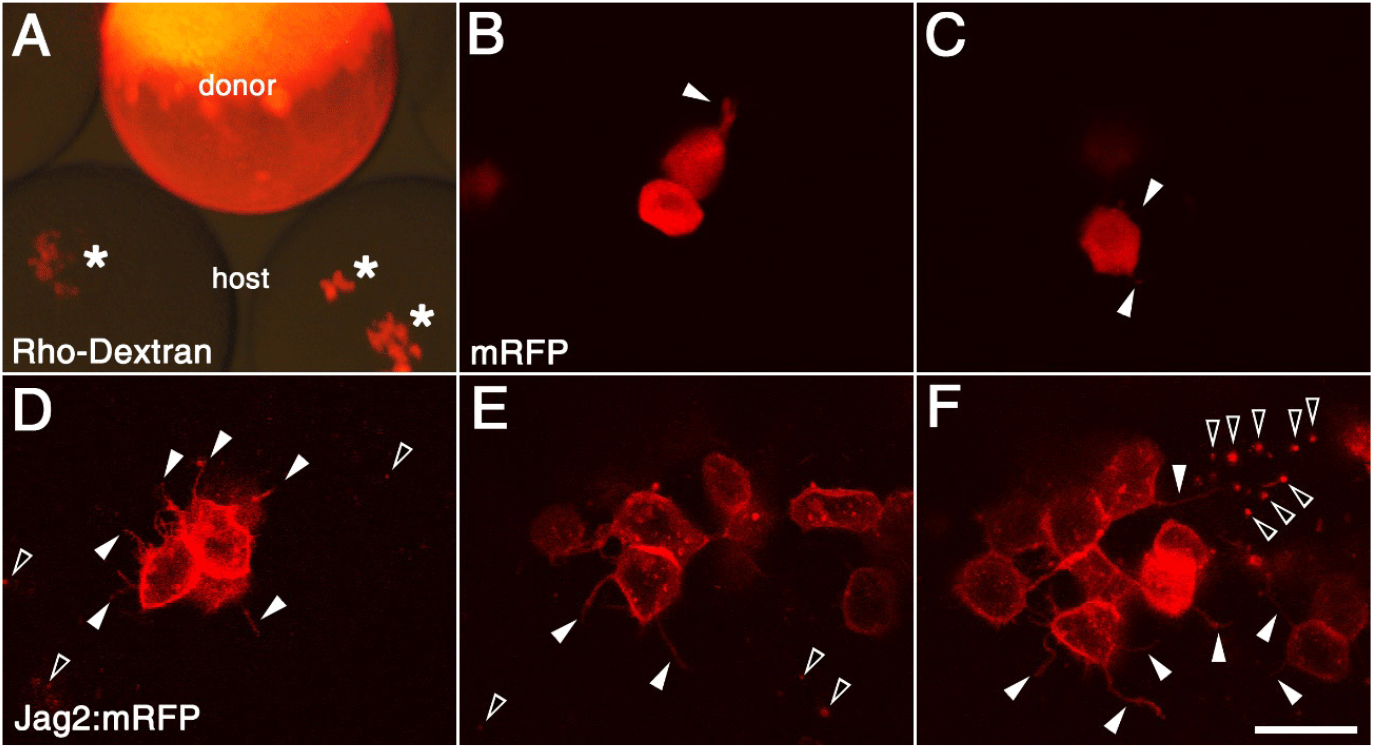
Cell transplantation experiments might be a useful way to test this possibility, therefore zebrafish fertilized eggs were microinjected with synthetic mRNAs (Fig. 4). In order to validate this experiment, a number of cells from the donor embryo, which had been injected with rhodamine dextran or synthetic mRNA of mRFP, were transplanted into host embryos (Fig. 4A–C). The majority of transplanted cells observed in the live donor embryos exhibited a rounded shape, with a small number of filopodia noted (Fig. 4B and C). In contrast, since Jagged2:mRFP was mainly expressed in the cell membrane, the transplanted cells were observed to be rounded in shape with a significant number of filopodia under fluorescence microscopy (Fig. 4D–F). Furthermore, the punctate fluorescent signals were observed both inside and outside the transplanted cells (Fig. 4F). While these intracellular red dots are thought to be endosomes, the extracellular punctate fluorescent signals could be the remnants of apoptotic cells or they might be EVs.
Next, a number of cells from the donor embryo, which had been injected with synthetic mRNA of Jagged2:mRFP and Mib:EGFP at 1-cell stage, were transplanted into host embryos (Fig. 5A). The host embryos were then incubated for 3 hours, after which time-lapse observations were conducted under the confocal microscope (Fig. 5). At the initial point in time, Jagged2:mRFP was predominantly expressed in specific areas of the cell rather than being uniformly distributed across the cell membrane within the transplanted cells (Fig. 5B). Furthermore, the presence of Jagged2:mRFP could also be observed in the extracellular space, manifesting as a few red dots (Fig. 5B). With the exception of a small number of extracellular green dots, the expression of Mib:EGFP in host embryos was observed to be almost identical to that of Jagged2:mRFP (Fig. 5C and D). The fluorescent dot inside the donor cell was thought to be an endosome (open arrowhead in Fig. 5B–D), while those outside the donor cells were thought to be EVs (arrowheads in Fig. 5B–D). Extracellular vesicles are membrane-bound particles that are naturally released from cells and act as messengers for intercellular communication (Doyle & Wang, 2019).
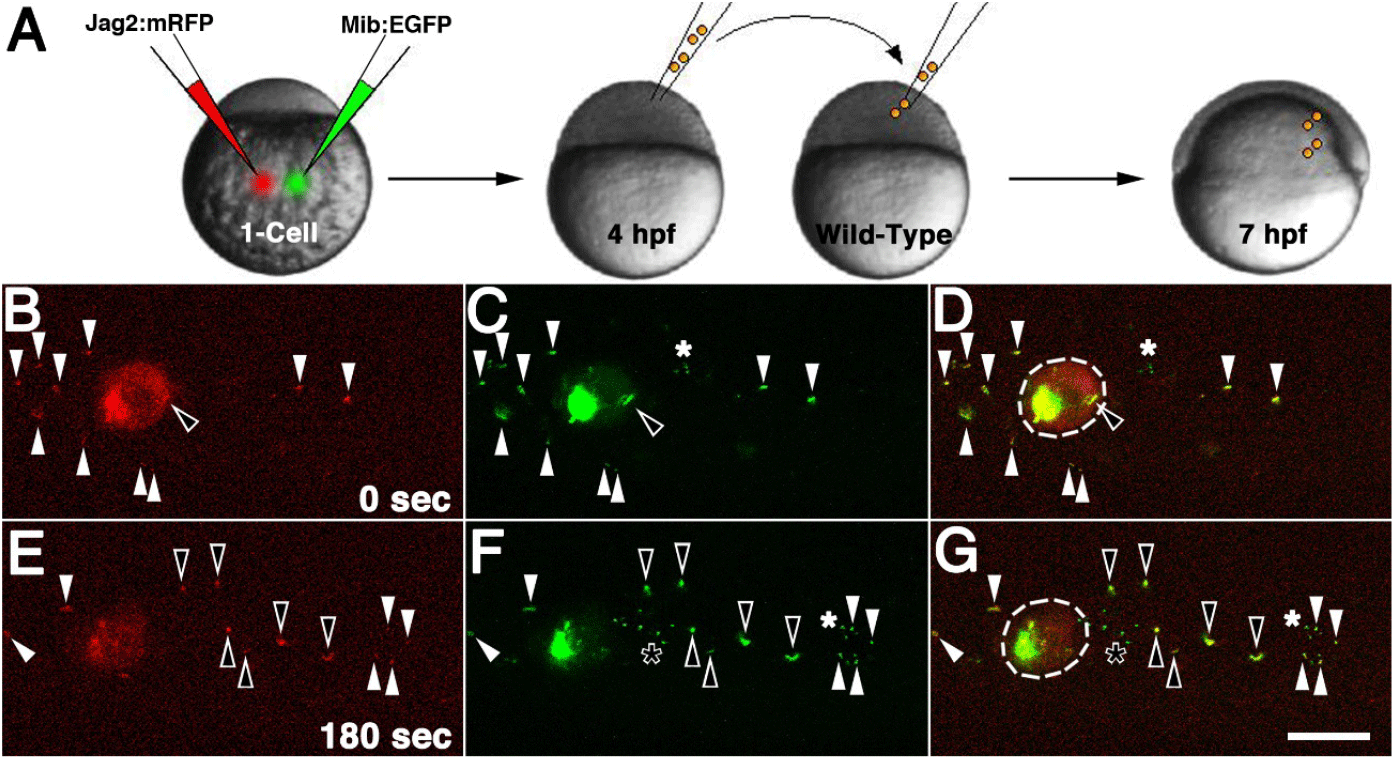
Three minutes after the initial time point, Jagged2:mRFP was found to be uniformly distributed across the donor cell, as opposed to being predominantly localized in specific regions (Fig. 5E). The few fluorescent dots of Jagged2:mRFP and Mib:EGFP expression that could be discerned in the extracellular space had undergone a marked displacement, moving further away from their initial locations (arrowheads in Fig. 5D and G). Furthermore, a fluorescent endosome was secreted outside the donor cell and divided into six newly formed EVs, which were observed to move along with existing EVs (open arrowheads in Fig. 5D and G). With the exception of a few newly formed green dots in the extracellular space, the expression of Mib:EGFP in the host embryo was observed to be almost identical to that of Jagged2:mRFP (Fig. 5F and G). The intensity of red fluorescence was also noted to decline in the Jagged2:mRFP-transplanted embryo when compared to the initial phase of observation (Fig. 5B and E). This decline was in contrast to the green fluorescence exhibited by the Mib:EGFP-transplanted embryo (Fig. 5C and F). Two observations were worthy of note. Firstly, the subcellular expression of Jagged2:mRFP was altered in the presence of Mib:EGFP and was predominantly observed in certain parts of the transplant cells. Secondly, Jagged2:mRFP-containing endosomes were observed to be released and migrate from the transplanted cell was observed, and all of these EVs were found to be Mib:EGFP-positive. Our results suggested that Jagged2 and Mib, which were found to bind together in vitro, were also present in the same location in vivo. Their complexes were found in large amounts in EVs, which implies that they may play a role in the Jagged-mediated Notch signaling pathway.
DISCUSSION
In this study, we investigated how Mib participates in the regulation of Jagged2. It was revealed that Mib-mediated ubiquitination of Jagged2 promotes proteasome-dependent degradation in undifferentiated P19 cells. Co-IP analysis showed that Mib physically interacts with Jagged2. In cell transplantation experiments, Jagged2:mRFP-positive cells formed elongated filopodia and secreted EVs. Co-expression of Mib:EGFP was shown to promote the formation of EVs containing Jagged2. These observations suggest that Jagged2-mediated Notch signaling can be classified not only a local induction mechanism but also as a long-range induction pathway.
Lateral inhibition mechanism is defined as a fundamental type of cell-to-cell interaction that occurs during animal development and is classified as one of the lateral signals (Chitnis, 1995). Previous studies have shown that Delta, a ligand of Notch, was ubiquitinated by Mib and internalized into the cell, thus executing a lateral inhibition mechanism (Itoh et al., 2003). These results suggested a model to explain how the endocytosis of ubiquitinated Delta in one cell could contribute to the activation of Notch signaling in neighboring cells (Itoh et al., 2003). In this study, it was demonstrated that Jagged2 was also ubiquitinated by Mib, thereby promoting its proteasome-dependent protein degradation. It was thought that, since the proteasomes are located both in the nucleus and in the cytoplasm, an E3 ubiquitin ligase would act in cytosolic protein quality control (Itoh et al., 2003). Therefore, it was assumed that Mib would act on the cytoplasmic domain of Jagged2. However, it was found that Mib did not bind directly to the intracellular domain of Jagged2, but only in the presence of the transmembrane domain. This observations presented in this study suggest two possible explanations. The first is that Mib might have a unique structural configuration that facilitates the recognition of the hydrophobic transmembrane domain of Jagged2. Secondly, there may be a membrane-specific adaptor that functions as a link between Mib and Jagged2. A yeast two-hybrid screen using Jagged2 provided evidence that a member of the AAA protein family, which resides in the cytoplasm but often binds to peroxisomal membranes to form heterotrimeric complexes, may play a role in Jagged2-mediated Notch signaling pathway (Ryu et al., 2024). Ubiquitination assay of Jagged2 by Mib using PEX1 knockout resource of undifferentiated cells will provide direct evidence of this possibility. In consideration of the results presented in this study, it could be deduced that the construction of a PEX1 KO model utilizing undifferentiated cells is imperative. In the RA-induced differentiation of the p19 cell line, proteasome-dependent degradation processes are limited. Proteolysis of Mib could also be observed in the differentiating P19 cell (Kong et al., 2018). It has been demonstrated that both fragments were generated in a retinoid pathway-dependent manner (Kong et al., 2018). Consequently, further experiments may be necessary to compare the ubiquitination of Jagged2 by Mib in both undifferentiated and differentiated PEX1 KO cells.
In an intracellular localization experiment performed to confirm the physical interaction of Mib with Jagged2, it was observed that Mib, which was mainly present in intracellular endosomes, mostly moved to the cell membrane when it was associated with Jagged2. Furthermore, Mib and Jagged2 were observed in large quantities in active filopodia of COS cells. This observation is reminiscent of the previous report that Delta expressed in primordial germ cells is transmitted to somatic cells located several diameters away through filopodia produced by the primordial germ cells (Yatsenko & Shcherbata, 2021). This is an example of the distant induction mechanism of Notch signaling. This observation was subsequently corroborated in cell transplantation experiments. When cells overexpressing Jagged2 were transplanted into host embryos, the majority of Jagged2 proteins behaved in a manner consistent with that observed in COS cells. A remarkable result observed during this cell transplantation experiment was the presence of fluorescent droplets containing Jagged2 in the extracellular space. Although the droplet might have been considered to be debris from apoptotic cells, subsequent time-lapse observations revealed that when cells containing both Mib and Jagged2 were transplanted, the droplets were likely to be an EV rather than debris. Extracellular vesicles are known to contain a variety of cargoes, including lipids, nucleic acids, and proteins, reflecting the cell from which they originate (Doyle & Wang, 2019). Extracellular vesicles, termed EVs, are classified into exosomes, microvesicles, and apoptotic bodies based on their formation and function (Doyle & Wang, 2019). Wang et al. (2019) reported that the Notch signaling pathway was activated following treatment with exosome derived from fetal dermal mesenchymal stem cells (FDMSCs). Subsequently, none of the ligands of the Notch pathway were detected in the exosome derived from FDMSCs except for Jagged1 (Wang et al., 2019). Conversely, endothelial cell-derived exosome has been shown to facilitate the delivery of Delta-like 4 (Dll4) protein to other endothelial cells, which results in the inhibition of Notch signaling and the subsequent loss of Notch receptors (Sheldon et al., 2010). The addition of Dll4 exosome has been demonstrated to confer tip cell properties to endothelial cells, thereby resulting in a high Dll4/Notch receptor ratio, low Notch signaling and filopodia formation (Sheldon et al., 2010). Despite the absence of regarding the activation or inhibition of the Notch signaling pathway by EVs containing Jagged2 and Mib, this study has elucidated, for the first time, that Mib-mediated ubiquitination of Jagged2 promotes its extracellular transmission via EVs. Furthemore, the observation that there might be a discrepancy in the degradability of ubiquitinated by Mib in pluripotent stem cells and differentiated cells provides a novel paradigm for the Jagged2-mediated Notch signaling. Taken together, these observations suggest the presence of a long-distance signaling mechanism within the Jagged2-mediated Notch signaling pathway.
