INTRODUCTION
The Hippo signaling pathway is an evolutionarily conserved pathway from Drosophila to humans. The Drosophila gene, called Hippo, and the mammalian homologs present in most body tissues and organs and regulates and promotes the growth of organs and tissues by inhibiting cell growth and promoting tissue regeneration-homeostasis and stem cell self-renewal and promoting cell death as needed (Udan et al., 2003; Huang et al., 2005; Zhao et al., 2010). Disruption of the Hippo signaling pathway in the imaginal discs of Drosophila is dependent on the proteins involved in the steroid reactive ecdysone (Ec) response. The Ec of Drosophila has a chemical structure such as estrogen and other steroid hormones found in humans (Delanoue et al., 2010). Taiman (Tai, NCOA3 orthologs) is an Ec receptor co-activator. Tai enhances the growth induced by Yorkie [Yki, yes-associated protein (YAP) orthologs], but the Tai form, Tai unable to bind Tai loss or Yki, inhibits tissue growth by Yki. This represents a Hippo / Ec pathway crosstalk in the form of the Yki-Tai complex that co-induces germline genes as part of a transcriptional program normally suppressed during somatic epithelia development (Zhang et al., 2015).
Recently, it has been shown that nemo-like kinase (NLK) can cross-talk with the Hippo pathway by phosphorylating an unknown new site of YAP. NLK transfers YAP serine 128 (S128) to the nucleus through dissociation of the 14-3-3 binding with YAP, promoting transcriptional activity (Hong et al., 2017; Moon et al., 2017). However, this is worth investigating as it has not been studied in the mammalian reproductive system and the precise mechanisms of signaling pathway crosstalk in endometrial cells that are dynamically altered by steroid hormones remain unclear.
The uterus is an important hormone responsive reproduction organ on mammals. Many uterine functions are under the regulation of ovarian steroid hormones, estrogen and progesterone, and uterine cell types respond to hormones in a differential manner (Hong & Choi, 2018; Lee et al., 2021). Ovarian Estrogen targets uterine epithelial cells, inducing proliferation and differentiation of these cells, whereas the proliferation of uterine stromal cells is under P4 regulation (Chung & Das, 2011). We have previously studied whether the Hippo signaling pathway is affected by intrauterine steroid hormones (Moon et al., 2019, 2022b). In a previous study, we demonstrated that STK4 and its downstream genes were induced or regulated by steroid hormones in the mouse uterus (Moon et al., 2019). Expression of STK4 and its downstream genes was increased and activated by Estrogen secretion (Moon et al., 2019). When STK4 is activated, the Hippo signaling pathway is turned on, and the nuclear release of YAP, phosphorylated at Ser127 by LATS1, proceeds normally (Moon et al., 2022a). However, YAP phosphorylated through estrogen regulation remains in the nucleus and acts within it (Moon et al., 2022a). This suggests that the normal Hippo signaling pathway is disrupted by other modulators, indicating crosstalk with signaling pathways other than the Hippo pathway via estrogen (Moon et al., 2022a). The purpose of this study is to elucidate whether NLK, which induces MAPK and Wnt signaling pathways, affects the regulation of YAP activity in the uterus, and whether their expression is regulated by hormones.
MATERIALS AND METHODS
All mouse experiments were performed on 7-week-old Institute of Cancer Research (ICR) mice provided by KOATECH (Pyeongtaek, Korea). Mice were housed under strict temperature and light control in the SPF animal room at the CHA Bio Complex (Seongnam, Korea). Mouse care and experimental and surgical procedures followed CHA University’s IACUC guidelines and were approved by CHA University’s Laboratory Animal Center Use Committee (Approval No. IACUC180128).
Vaginal cell samples from mice were smeared on glass slides and then dried in a 60°C heat block. Slides were immersed in Giemsa solution and left for 1 min. After that, they were soaked in eosin for 1 minute, but there was no significant difference when observed under a microscope, so they were excluded from the staining process. After Giemsa staining, the solution was immersed in distilled water to remove the Giemsa solution remaining on the slide, and after wiping it with a kimtech wiper (Yuhan-Kimberly, Seoul, Korea), it was immediately observed under a microscope.
At least 5 7-week-old ICR mice per estrogen treatment time were ovariectomized (OVX). Anesthetized with Avertin (8–10 mg/mouse, Sigma-Aldrich, St. Louis, MO, USA), the back of the anesthetized mouse was incised and wiped with alcohol. A skin incision was made in the depression along the spine. The ovaries and fallopian tubes were removed and removed, and the remaining tissue was put back into the abdominal cavity. The incised skin was sutured with thread. There was a recovery period of 10 days after OVX. After the recovery period, β-estradiol (E2; Sigma-Aldrich) was injected into the mice at 200 ng/mouse (McLaren, 1968; Lee et al., 2003) and progesterone (P4; Sigma-Aldrich) at a concentration of 2 mg/mouse (Lloyd, 1937). For the assessment of responsiveness to hormones, estrogen receptor (ER) antagonist ICI 182,780 (ICI, Sigma-Aldrich) (500 μg/mouse) and progesterone receptor antagonist RU486 (RU; Sigma-Aldrich) (1 mg/mouse) were pre-injected 30 minutes prior to hormone injection.
Rabbit polyclonal anti-NLK (Alexa 555) antibody was purchased from Bioss (bs-10420R-A555). Rabbit polyclonal anti-YAP (phospho-Ser128) was generated using recombinant YAP peptide PQHVRAHS<pS>PASLQLG and affinity purified (AbClon, Seoul, Korea) (Moon et al., 2017). The sampled tissues were fixed with 4% paraformaldehyde. The paraffin block tissue was sectioned to a thickness of 5 μm using a microtome (Macroteck, Yongin, Korea) and placed on a slide glass. Deparaffinization was performed as follows; 100% ethanol, 95% ethanol, 70% ethanol, 50% ethanol, and running water for 5 minutes each. Slides were placed in a container with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). The vessel was boiled for 40 minutes in an antigen retrieval steamer (IHCWORLD, Irvine, CA, USA). The slides were washed once in distilled water for 5 minutes and washed 3 times with 0.05% phosphate-buffered saline with tween-20 (PBST) for 5 minutes. A hydrophobic barrier was drawn around each tissue on the slide using an ImmEdge pen (Vector Lab, Road Burlingame, CA, USA). Slides were placed in a humidified chamber and then dispensed with 5% goat serum blocking buffer (0.05% PBST and 4% BSA). The chamber was incubated at room temperature for 4 h, then treated with primary antibody and incubated at 4°C overnight. Slides were washed 3 times for 5 minutes in PBST, then treated with secondary antibody Alexa-Fluor antibody 488 (Invitrogen, Waltham, MA, USA) and incubated for 1 h at room temperature. Slides were washed 3 times with PBS and treated with 4’,6-diamidino-2-phenylindole (DAPI) (Life Technologies, Carlsbad, CA, USA) for 10 minutes at room temperature. After washing the slides 3 times for 5 minutes with PBS, a drop of mounting medium (Dako, Carpinteria, CA, USA) was dropped on the slide and covered with a glass coverslip. Fluorescence staining was observed with a confocal microscope LAS X (Leica Microsystems, Wetzlar, Germany). For immunofluorescence, tissues from at least three independent biological replicates (n=3) per group were sectioned and processed.
For Immunohistochemistry, Mouse polyclonal anti-NLK antibody was purchased from Santa cruz (sc-48361, Santa Cruz Biotechnology, Dallas, TX, USA). Antibodies not mentioned herein have already been described previously. After deparaffinizing the paraffin block slides, the sections were incubated with 3% hydrogen peroxide (H2O2) in methanol for 10min at room temperature to eliminate endogenous peroxidase activity. Slides were washed once for 5 minutes in running water and three times for 5 minutes in PBST buffer. After antigen retrieval, the blocking solution was treated, and the primary antibody was attached and incubated overnight at 4°C. After treatment with the secondary antibody for 1h, DAB solution (Vector Laboratory, Newark, CA, USA) was dropped on the slide and waited until the desired signal appeared. After the DAB reaction, the slides were immersed in 1X phosphate buffer (0.1 M PB, pH 7.4). The slides were washed once in running water for 5 minutes. The slides were counterstained with hematoxylin, soaked in supersaturated sodium bicarbonate for 1 minute to change the hematoxylin stain to blue, and then washed again in running water for 5 minutes. Slides were rehydrated with ethanol and xylene and a drop of permount mounting medium (Thermo Fisher Scientific, Waltham, MA, USA) was added to the slides and covered with a glass coverslip. For immunohistochemical analyses, tissues from at least three independent biological replicates (n=3) per group were sectioned and processed.
Total RNAs were extracted from mouse uteri using RNeasy total RNA isolation kit (Qiagen, Hilden, Germany) according to the manufacturer’s instruction. The total RNAs (2 μg) were reverse-transcribed to synthesize complementary DNA (cDNA) using SuperScript® III First-Strand Synthesis System (Life Technologies) following the manufacturer’s instruction.
Conventional quantitative real-time polymerase chain reaction (RT-PCR) was performed using a ProFlex PCR System and/or a SimpliAmp Thermal Cycler (Thermo Fisher Scientific). The standard thermal cycling parameters were as follows: denaturing at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The products were loaded and analyzed in 2% agarose gel.
Quantitative RT-PCR analysis was performed using the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Amplification was carried out using iQ™ SYBR® Green Supermix (Bio-Rad). Cycling conditions for analysis were as follows; Denaturation at 95°C for 15 seconds, annealing at 60°C for 15 seconds and at 72°C for 20 seconds, 50 cycles. Comparative CT (ΔΔCT) method was used for relative gene expression analysis (Livak & Schmittgen, 2001). Relative level value of gene expression was normalized to the relative amounts of Rpl7 as reference gene. The primer sequences are listed in Table 1, along with the gene symbol for each primer.
Mouse monoclonal anti-β-actin antibody (C-4) was obtained from Santa Cruz. Rabbit Polyclonal anti- Dexras1(RASD1; ab78459) was purchased from Abcam. Other antibodies not mentioned are described in Immunostaining methods.
Proteins were obtained by pulverizing the sampled uterine tissue with a homogenizer and using a pro-prep reagent containing NaVO4, β-glycerolphosphatase, and NaF. After SDS-PAGE and semi-transfer, the polyvinylidene difluoride (PVDF) membrane was blocked with ProNA™ phospho-block solution (TransLab, Seoul, Korea) at room temperature for 1 hour. Primary antibodies were diluted in blocking solution and incubated overnight at 4°C and/or 2 h at room temperature. After washing 3 times for 5 minutes with 0.05% PBST, an appropriate HRP-binding secondary antibody (Invitrogen) was incubated for 1 hour at room temperature. Then wash 5 times for 3 minutes with 0.2% PBST. ECL reaction was performed using Western ECL FAMTO Kit (LPS Solution, Seongnam, Korea). Relative expression was analyzed by the ChemiDoc XRS system (Bio-Rad).
HEK293T cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS. YAP mutants (S127F and S128A) were generated by site-directed mutagenesis, substituting serine residues with phenylalanine and alanine, respectively. Plasmids were transfected into HEK293T cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Cells were harvested 24 h post-transfection for Western blot analysis.
RESULTS
Prior to examining the expression of NLK in utero, we assessed the relative levels of Nlk transcripts during the estrous cycle using RT-PCR and q-PCR. As shown in Fig. 1A and B, Nlk expression was dynamically regulated during the estrous cycle divided into four phases (P, proestrus; E, estrus; M, metestrus; D, diestrus). Quantitative RT-PCR analysis revealed that Nlk mRNA levels were significantly lower in the estrus and metestrus stages compared to proestrus (Fig. 1B). Differential NLK protein level during the estrous cycle was confirmed by Western blotting analysis (Fig. 1C). The NLK protein expression level showed a slightly different appearance from the mRNA pattern. NLK was expressed similarly in all stages and showed a slight increase in the estrus stage. The protein expression level of YAP S128, which is known to be phosphorylated by NLK, was found to be similar to that of NLK. As shown in Fig. 1D, vaginal smear assay identified each phase of the estrous cycle. Next, the expression location and expression intensity of NLK and YAP S128 in the uterus during the estrous cycle were investigated. NLK is known to regulate phosphorylation of YAP competitively with LATS1 (Hong et al., 2017; Moon et al., 2017). As shown in Fig. 1E, immunofluorescence analysis of at least three independent biological replicates (n=3) confirmed that the co-localization of NLK and YAP S128 is dynamically regulated during the estrous cycle. Notably, the stage-specific nuclear translocation of these proteins was consistently observed across all examined samples, representing a robust and reproducible phenotype in response to physiological hormonal shifts.
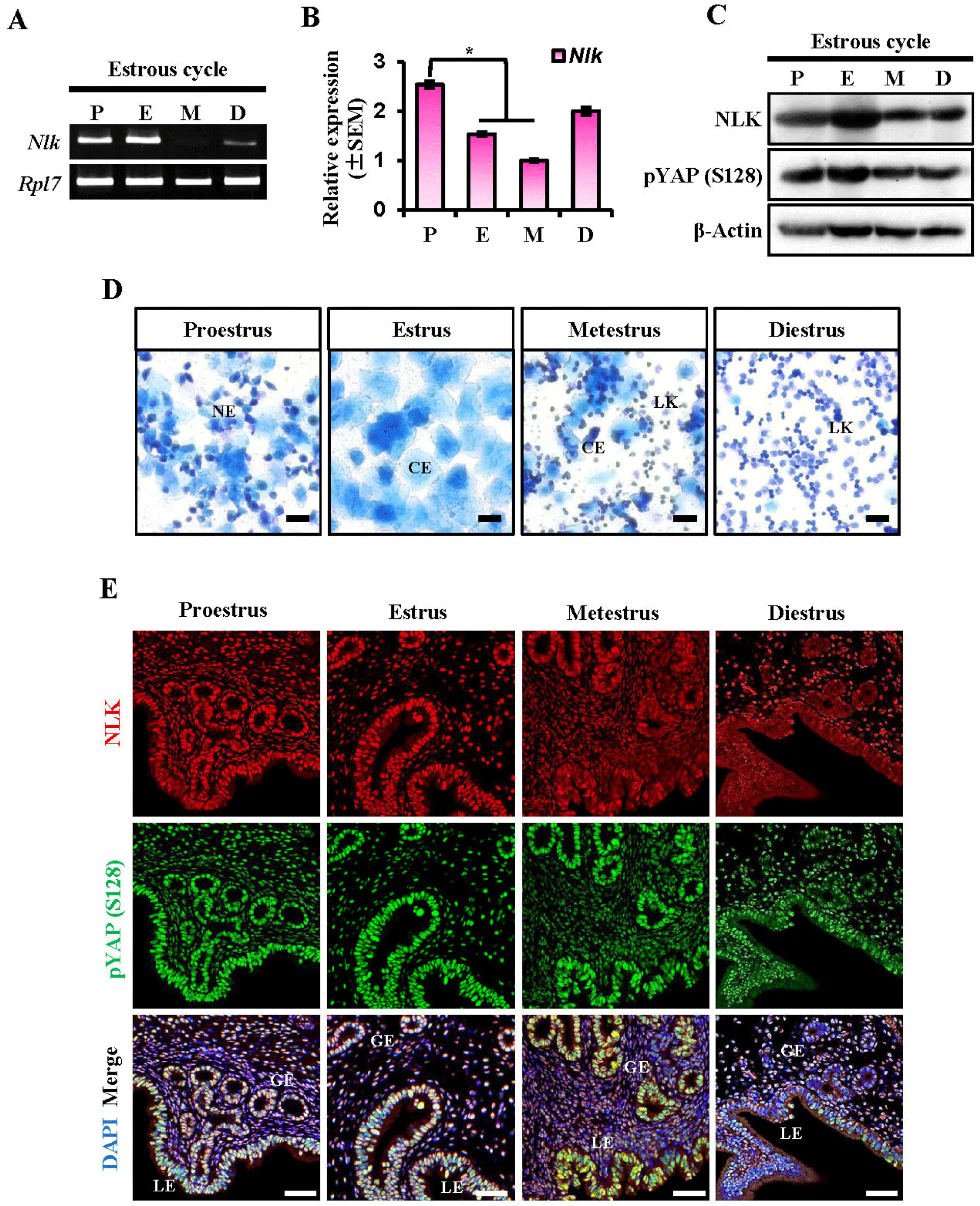
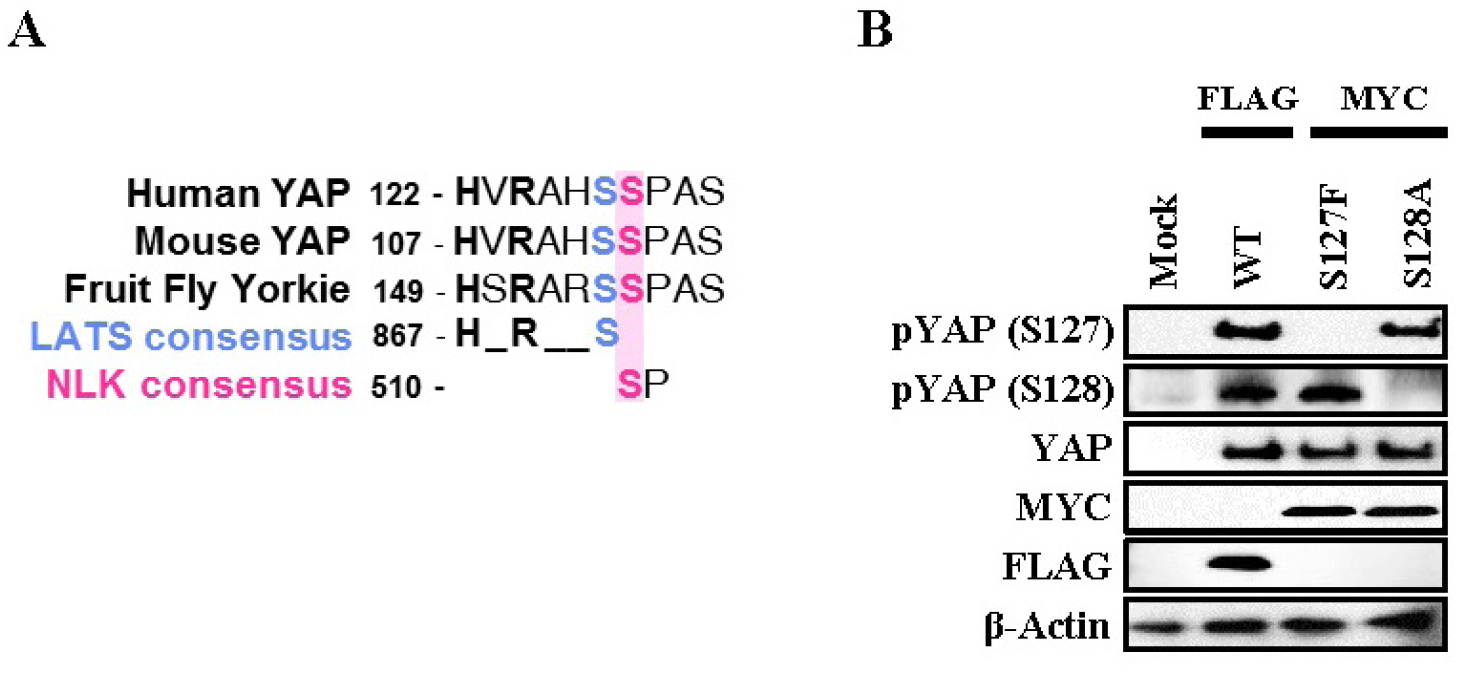
NLK is located in the nuclei of luminal epithelial cells and glandular epithelial cells during the proestrus, estrus, and metestrus stages, and then moves to the cytoplasm of the luminal epithelial cells during the diestrus stage. YAP S128 is located in the nuclei of luminal epithelial cells and glandular epithelial cells at all stages, and their co-expression is strongest at metestrus stage. While NLK protein was also detectable in the stromal compartment, its expression remained relatively stable across the estrous cycle, whereas the epithelial cells showed dynamic stage-dependent localization. The expression of NLK and YAP S128 was relatively weak during the diestrus phase among the four estrous stages. As shown in Fig. 2, it was confirmed that an antibody against YAP S128 specifically recognizes the S128 site by constructing a point mutation vector for YAP S127 and YAP S128.
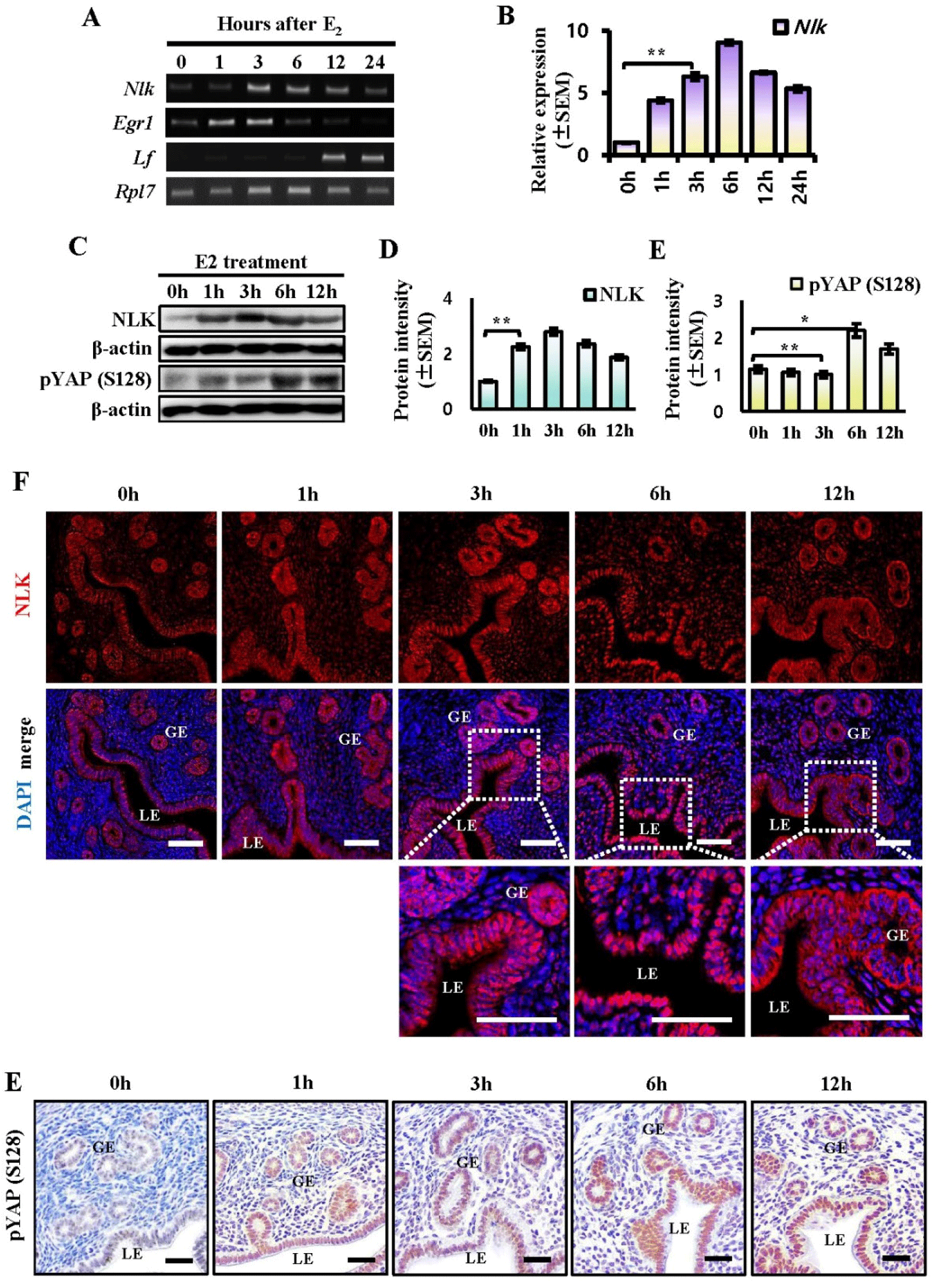
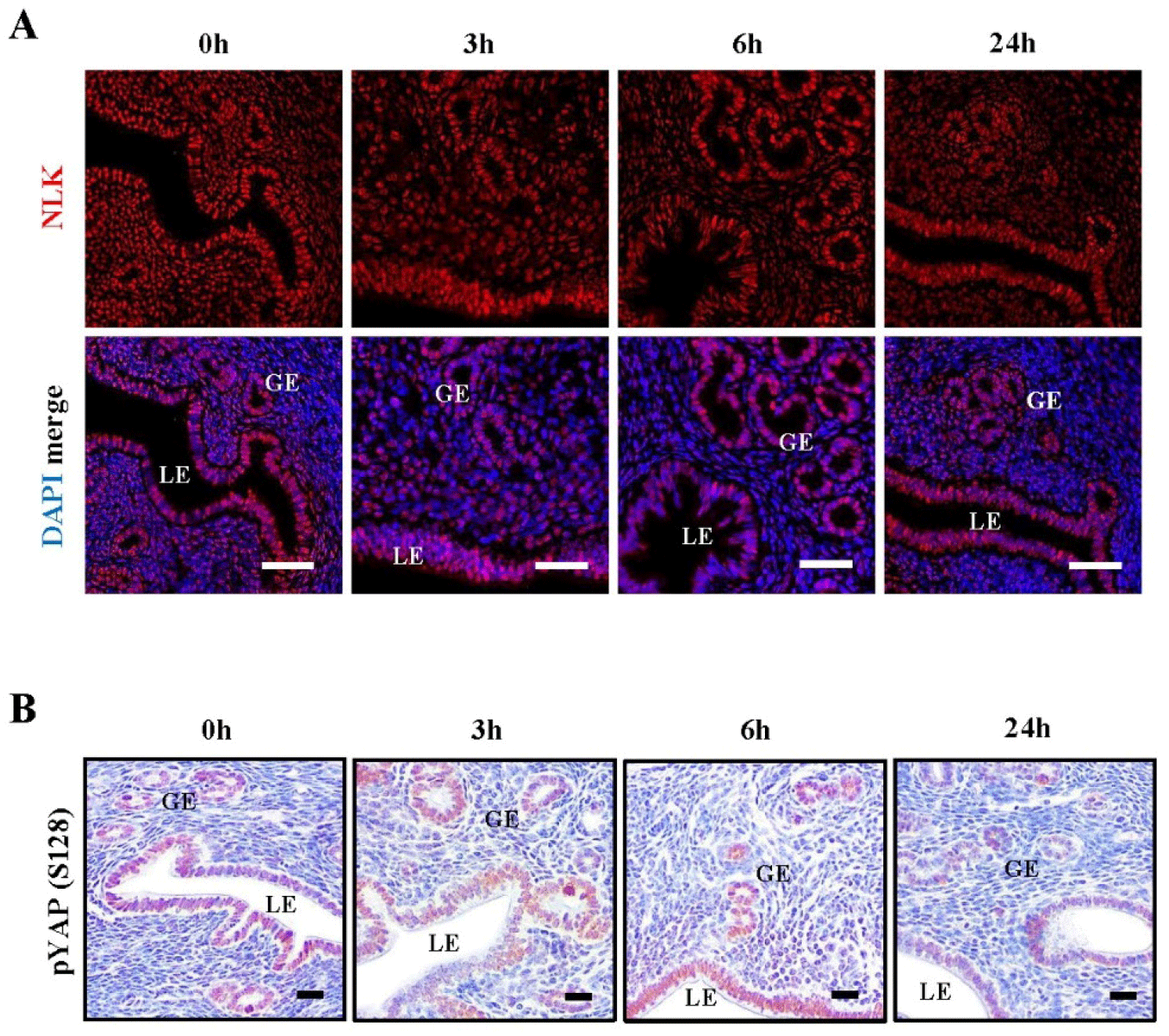
Since we previously confirmed that STK4 expression is affected by estrogen during the estrous cycle, we investigated whether NLK is also affected by estrogen (Moon et al., 2019). We used ovariectomized mice to confirm the estrogen-induced NLK expression response. RT-PCR and q-PCR analyzes showed that the expression of NLK transcripts changed time-dependently after estrogen injection (Fig. 3A and B). The Nlk transcript levels showed a significant induction starting from 3 h and maintained an elevated state until 6 h after E2 treatment (Fig. 3B). We confirmed the expression of lactoferrin (Lf), a late estrogen response gene, and early growth response 1 (Egr1), an early estrogen response gene, to demonstrate estrogen response in ovariectomized mice (Jeong et al., 2015). Then, NLK protein expression and YAP S128 phosphorylation were observed every hour after hormone injection in OVX mice. Western blot analysis showed that the expression of NLK and YAP S128 was altered in a time-dependent manner (Fig. 3C). NLK increased from 1 hour after estrogen injection, peaked at 3 hours, and decreased after 6 hours. The expression of YAP S128 was found to increase from 6 hours after estrogen injection. Immunofluorescence shows that NLK is regulated by endometrial estrogen. NLK coexists in the cytoplasm and nucleus of LE and GE and was found to be confined to the nucleus 6 hours after estrogen treatment. After 12 hours of estrogen treatment, it was confirmed that they were present in the cytoplasm and nucleus again (Fig. 3F). Immunochemical staining showed that YAP S128 expression was increased by estrogen in the uterus. YAP S128 expression showed a time-dependent increase in the nuclei of LE and GE (Fig. 3E). These results suggest that NLK and YAP S128 expressions are regulated by estrogen during the estrous cycle.
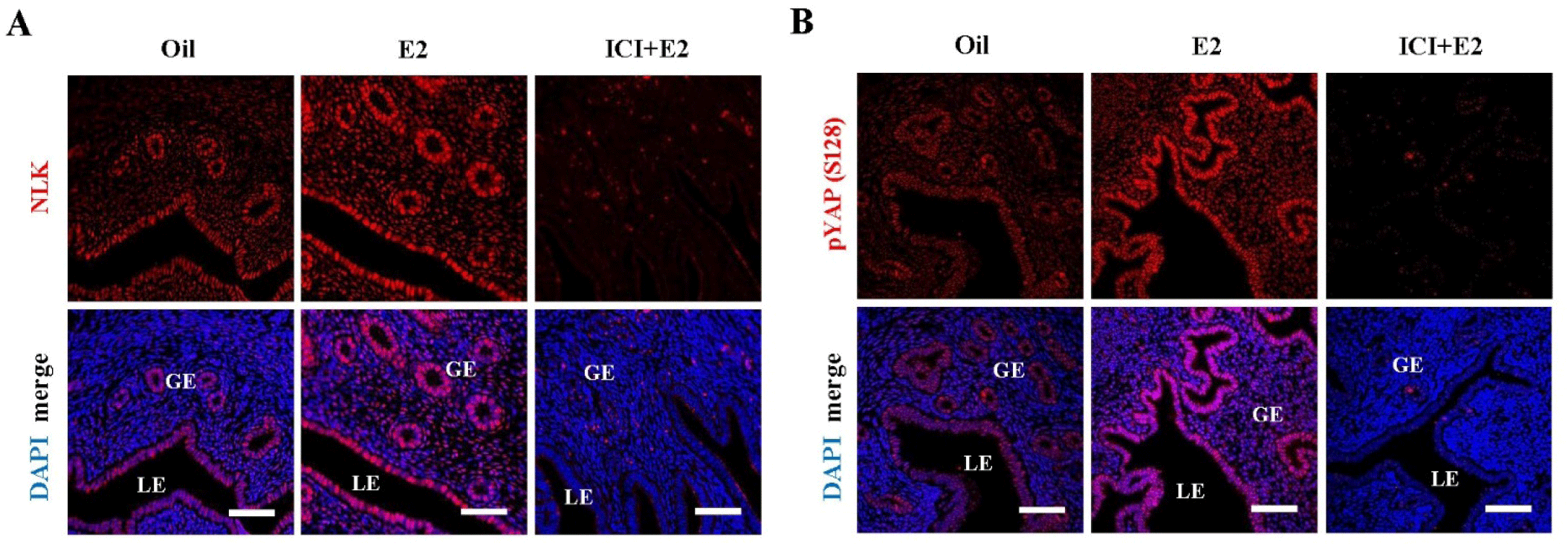
To determine whether expression of estrogen-induced NLK and YAP S128 is mediated by the ER, ovariectomized mice were pretreated with the ER antagonist ICI 182,780 (ICI) 30 minutes prior to estrogen injection. Protein expression of NLK and YAP S128 were investigated 6 hours after estrogen injection. Immunofluorescence staining confirmed that estrogen increased the expression of NLK and YAP S128 in LE and GE. Estrogen-induced increases in NLK and YAP S128 expression were significantly prevented by ICI (Fig. 4A and B). These results suggest that estrogen induces the expression of NLK and YAP S128 by ER-mediated pathways in the LE and GE of the uterus.
After confirming the response by estrogen, we also wanted to investigate the response by progesterone. Immunofluorescence staining results show that there is no difference in the expression level of NLK by progesterone. It was confirmed that NLK was still present in the endometrium at a similar intensity even after a period after progesterone treatment (Fig. 5A). As a result of immunochemical staining, the expression of YAP S128 after progesterone injection decreased 6 hours after progesterone injection. This decrease appeared to increase slightly again 24 h after progesterone injection (Fig. 5B). During the mouse estrous cycle, estrogen and progesterone are alternately secreted by the ovaries and act either singly or jointly in endometrial remodeling (Nilsson et al., 2015). Taken together, the differential responses of NLK and YAP S128 to estrogen and progesterone suggest that coordinated hormonal interactions may play a critical role in regulating their expression.
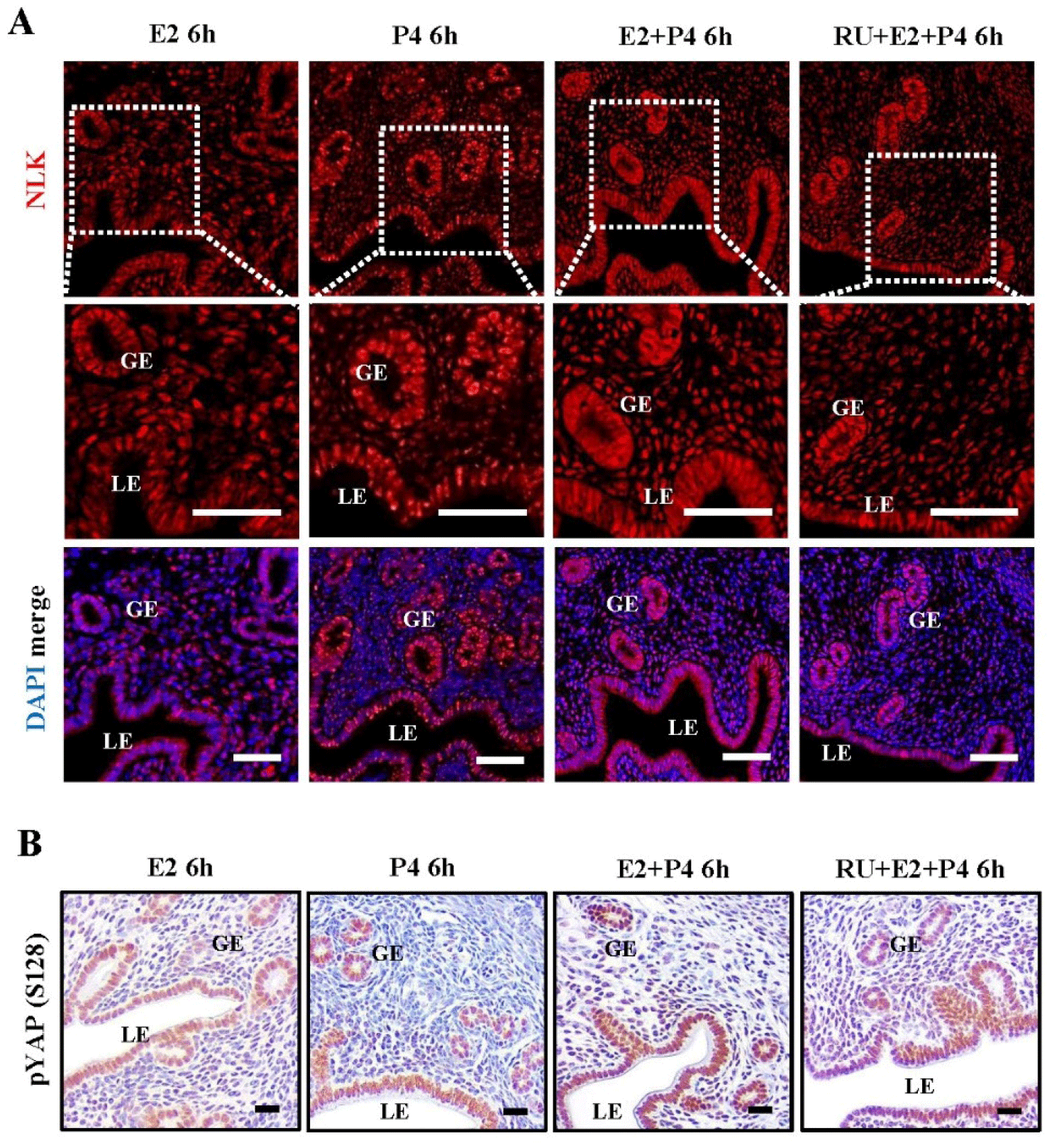
Our results showed that both NLK and YAP S128 showed changes after 6 hours of estrogen and progesterone injection, so we focused on the 6 hours and investigated the simultaneous effects of estrogen and progesterone. Immunofluorescence staining showed that the expression site of NLK was different when estrogen and progesterone were present together. In the case of estrogen or progesterone alone treatment, NLK was present in the nucleus of LE and GE, but it was confirmed that it was present in the nucleus and cytoplasm of LE and GE at the same time when combined treatment (Fig. 6A). As a result of immunochemical staining, YAP S128 showed stronger expression when administered in combination with estrogen and progesterone than with estrogen or progesterone alone. When RU486 (RU), a progesterone receptor antagonist, was administered 30 minutes prior to administration of estrogen and progesterone, the expression of YAP S128 was decreased compared to the hormonal combination treatment (Fig. 6B). These results imply that NLK and YAP S128 in the endometrium are regulated and dynamically changed by the single and synergistic actions of these hormones.
DISCUSSION
In this study, we investigated the novel gene, NLK and YAP S128 involved in the Hippo signaling pathway in the uterus. We found that the expression of NLK and YAP S128 changes the expression site or extent of expression in the endometrium during the estrous cycle (Fig. 1). Furthermore, we demonstrated that regulation of its expression proceeds through estrogen and its receptors (Figs. 3 and 4). We have shown that these responses are triggered and regulated in uterine epithelial cells, suggesting that their expression plays a role in uterine dynamics during the estrous cycle, as does the Hippo signaling pathway.
The evolutionarily conserved Hippo pathway from nematodes to humans was discovered in Drosophila studies to identify genes involved in organ size regulation (Zeng & Hong, 2008). Hippo-STK3/4, Warts-LATS1/2, and Yorkie-YAP are key components of the Hippo signaling pathway that, when mutated, lead to dramatic overgrowth of mutant-bearing tissues (Harvey et al., 2003; Udan et al., 2003; Wu et al., 2003; Huang et al., 2005). So far, the well-known hippo pathway is a phosphorylation cascade leading to STK3/4, LATS1/2, and YAP. Activation of this signaling pathway leads to STK3/4-mediated direct phosphorylation of LATS1/2 followed by LATS1/2-mediated direct phosphorylation of YAP S127. YAP S127 interacts with 14-3-3 protein to promote cytoplasmic maintenance and leads to β-TrCP-mediated proteasome degradation of YAP. S127 phosphorylation of YAP is known to be essential for interaction with 14-3-3, and β-TrCP-mediated proteasome degradation is accomplished by LATS1/2-mediated S397 phosphorylation of YAP (O’Neill & Kolch, 2005; Zhao et al., 2007, 2010). Conversely, dephosphorylation of YAP moves to the nucleus and activates genes involved in cell proliferation and tissue hyperproliferation through interaction with TEAD1-4 transcription factors (Zhao et al., 2008; Nishioka et al., 2009; Lamar et al., 2012). In addition to LATS1/2, AKT was confirmed to be involved in YAP S127 phosphorylation. It is known that AKT induces 14-3-3 interaction through S127 phosphorylation of YAP and regulates apoptosis (Strano et al., 2001; Kawamura et al., 2013). Therefore, it is believed that YAP S127 phosphorylation, including LATS1/2, is an important process essential for regulating the transcriptional activity of YAP.
Phosphorylation of YAP was taken to a new level by NLK. NLK is known to regulate the activities of several transcription factors, including the Wnt pathway, the Notch pathway, and the FOXO3 pathway, which are key to various signaling pathways (Ishitani et al., 1999, 2010; Kim et al., 2010). NLK has been shown to be localized and activated in the nucleus (Ishitani et al., 2011). When cells are at low density, nuclear NLK phosphorylates YAP S128 and prevents YAP S127 and S397 phosphorylation by LATS1/2, allowing YAP to act as a transcriptional activator in the nucleus (Moon et al., 2017). YAP S128 is located in the 14-3-3 binding region (Lee & Yonehara, 2012; Zhao et al., 2014; Yuan et al., 2015). Other studies suggest that YAP S128 phosphorylation by NLK is incompatible with 14-3-3 binding and consequently inhibits 14-3-3 binding of YAP and induces its accumulation in the nucleus (Hong et al., 2017).
In this study, we observed the dynamic nuclear co-localization of NLK and p-YAP (S128) in endometrial epithelial cells during the estrous cycle and following estrogen treatment. Although we did not directly measure the expression of YAP/TEAD target genes, it is widely established that NLK-mediated S128 phosphorylation is a prerequisite for YAP’s nuclear retention and transcriptional co-activation by disrupting its interaction with 14-3-3. Consequently, the induction of nuclear p-YAP (S128) by estrogen functions as a robust biochemical indicator of activated Hippo pathway crosstalk within the uterine epithelium. The present findings provide a necessary foundation for understanding how hormonal signals modulate uterine dynamics through non-canonical YAP regulation. When estrogen and progesterone coexist, they are more expressed through cooperative action. Estrogen and progesterone are secreted at different times but are related. It is known that an increase in the secretion of progesterone from the corpus luteum of the ovary gives a positive feedback effect of estrogen that induces a GnRH surge before ovulation (Caraty & Skinner, 1999). Estrogen action through ER in the uterus upregulates progesterone receptor expression, and the interaction of these hormone signaling pathways is important for uterine function and fertility (Marquardt et al., 2019). In this context, the observations that NLK and p-YAP (S128) levels are further modulated by the combined treatment of E2 and P4 suggest a cooperative regulation between these two hormonal pathways. The joint coordination of the spatio-temporal dynamics of the NLK-YAP axis by E2 and P4 prepares the endometrium for physiological changes. To investigate this hormonal regulation, we utilized ICI 182,780 to inhibit ER signaling. Although ICI 182,780 is a pan-antagonist that blocks both ERα and ERβ, it is well-documented that ERα is the primary mediator of estrogen-induced proliferation and signaling in the uterine epithelium (Winuthayanon et al., 2010). While our results demonstrate that the NLK-YAP axis is regulated via an ER-dependent mechanism, future studies using subtype-specific models would be necessary to definitively distinguish the individual roles of each receptor.
In conclusion, our results indicate that NLK and YAP S128 are regulated by estrogen and progesterone in the endometrium. These findings offer novel insights into Hippo signaling pathways in uterine dynamics.
