INTRODUCTION
Mesenchymal stem cells (MSCs) are a heterogeneous cell population characterized by plastic adherence, self-renewal capabilities, and multi-differentiation potential into osteoblasts, adipocytes, chondrocytes, and other cells. They are considered as good candidates for therapeutic cells due to limited tendency to produce tumors and immunologically privileged nature (Uccelli et al., 2008). It has been shown that MSCs migrate to injured tissues, then differentiate into tissue-specific cells or promote regeneration by secretion of bioactive factors. Their therapeutic effects have been demonstrated in various disease models such as heart, brain, liver, spleen, and lungs (Bianco et al., 2008). Bone marrow-derived stem cells (BM-MSCs) are the most well-known and characterized MSCs, but their invasive procedure lead to a limited availability for clinical application. Multipotent stem cells present in adipose tissue, adipose-derived stem cells (ADSCs), are promising stem cell population, since human adipose tissue is ubiquitous and easily obtained in large quantities with little donor site morbidity or patient discomfort. The use of autologous ADSCs as both research tools and as cellular therapeutics is feasible and has been shown to be both safe and efficacious in preclinical and clinical studies of injury and disease (Mizuno et al., 2012).
Intravenous infusion of MSCs is the most practical route of administration for many therapeutic objectives. It is well known that engrafted cells home to injured tissue by various signals such as chemokines, integrins and matrix metalloproteinases (MMPs) (Sarkar et al., 2011). For example, MSC migration at the fracture site is well known to be time- and dose-dependent and is exclusively CXCR4-dependent (Granero-Moltó et al., 2009). Integrins are heterodimeric transmembrane protein receptors consisting of noncovalently linked α and β subunits (Prowse et al., 2011). They affect many processes, including cell-cell adhesion, cell-ECM adhesion, cellular differentiation, proliferation, motility, and signal transduction. Human BM-MSCs are known to express a broad range of integrins in their undifferentiated state such as α1-6, 10, 11, V and β1-8 (Brooke et al., 2008). They show a dramatic, though transient, increase in the level of α5 integrin on day 7 of osteogenesis and an increase in α6 integrin expression throughout adipogenesis, and presentation of RGD peptides, which is bound by multiple integrins, was shown to be required to support long-term viability of hMSCs (Frith et al., 2012). Knockdown of the α6 integrin subunit in BM-MSCs significantly reduces capillary sprouting, and causes their failure to associate with the nascent vessels (Carrion et al., 2013). Adhesion, homing, lodging and retention of haematopoietic stem cells and progenitors in the bone marrow have also been shown to occur through Rab5-dependent post-translational regulation of β1/β2 integrins (Taniguchi Ishikawa et al., 2013). MMPs are proteolytic enzymes that depend on binding with a Zn2+ ion for their catalytic activity. Two groups of MMPs are known, soluble, and membrane anchored. Most of the soluble MMPs are secreted as inactive prozymogens and activated by other proteases through cleavage of their N-terminal prodomains. The proteolytic activity of MMPs in the extracellular space is controlled by a series of natural endogenous inhibitors, with TIMPs representing the major group of proteins involved in this inhibition. Migration of BM-MSCs through bone marrow endothelium was shown to involve MMP-2, and the migration was decreased by TIMP-3 (De Becker et al., 2007). The inflammatory cytokines TGF-beta1, IL-1beta, and TNF-alpha up-regulate MMP-2, MT1-MMP, and/or MMP-9 production in MSCs, resulting in a strong stimulation of chemotactic migration through ECM. (Ries et al., 2007). Collectively these studies demonstrate that migration of MSCs could be regulated by cytokines, MMPs and TIMPs.
Fetal bovine serum (FBS) used in cell expansion protocols provides vital nutrients, attachment factors, toxin scavengers and growth factors. However, it is unsuitable for therapeutic purposes due to the ill-defined nature, high lot-to-lot variable composition, the risk of transmitting infectious agents and immunizing effects. Various supplements have been developed to replace FBS such as a fully chemically defined medium and pooled human AB serum (Tateishi et al., 2008; Bieback et al., 2012). Human factors purified from serum or platelets have also been suggested as alternatives for clinical scale manufacturing (Bieback et al., 2010). Previously, we have observed that human eyelid adipose-derived stem cells (HEACs) cultured in medium containing human serum (HS) readily formed aggregation and the aggregated cells followed by disaggregation in FBS medium maintained their stem cell characteristics (Song et al., 2012).
In this study, we aimed to investigate the expression of cell-adhesion molecules and MMPs in cell aggregates formed by high-density culture of HEACs in the presence of HS. We observed that the expression of integrin α2, α2B, αX, MMP1 and MMP9 genes in HS-agg of HEACs, and MMP9 enzymatic activity remarkably increased during culture. These results suggest that integrins and MMPs might be involved in the HS-induced aggregation of HEACs.
MATERIALS AND METHODS
Human serum (HS) was purchased from Millipore (Millipore, Temecula, CA). It was inactivated at 56°C for 30 min and aliquots were stored in 50-ml tube at –20°C until use. All reagents were purchased from Sigma unless stated otherwise.
Human eyelid adipose tissues were obtained from ten patients aged between 24 and 56 years (mean age, 36.7± 4.7 years) undergoing cosmetic surgery in local hospital in Seoul with informed consent. About 0.5 g of tissue specimen was usually obtained by an individual. All experiments were approved by Institutional Review Board of Seoul Women’s University. Cells were prepared as described previously (Kang et al., 2009). Tissues were washed with Dulbecco’s phosphate buffered saline (DPBS, Gibco, Grand Island, NY) containing Antibiotic-Antimycotic (Gibco) and treated with 1× volume of 0.15% type I collagenase (Gibco) in DPBS for 30 min at 37°C with gentle stirring. Then an equal volume of Dulbecco’s modified Eagle’s medium-low glucose type (5.5 mM; DMEM, Gibco) supplemented with Antibiotic-Antimycotic (Gibco), 3.7 mg/ml sodium bicarbonate and 10% FBS (Gibco) was added to neutralize the enzymatic activity. After centrifugation at 3,000 rpm for 10 min, the supernatant was removed and the remaining cell pellet was washed several times with DMEM. For the routine culture, cells were plated in 25-cm2 culture flask (Nunc, Rockilde, Denmark) containing 5 ml of DMEM supplemented with Antibiotic-Antimycotic, 3.7 mg/ml sodium bicarbonate, and 10% FBS. Cells were cultivated at 37°C with 5% CO2. The medium was changed twice a week. HEACs at passage 3 (p3) to p6 were used in this study.
HEACs were seeded in 6-well plate (Nunc, Shanghai, China) at a density of 2×105 cells/well. Cells were cultivated with 5 ml of DMEM containing 10% of either FBS or HS. Morphological change was observed every day during culture until 20 days. Around the time of aggregation, cells were treated with Hank’s balanced salt solution containing 0.125% trypsin and 1mM EDTA for 2 min at 37°C. Then DMEM containing 10% FBS was added to stop the enzymatic activity. After several washing, cell number was counted.
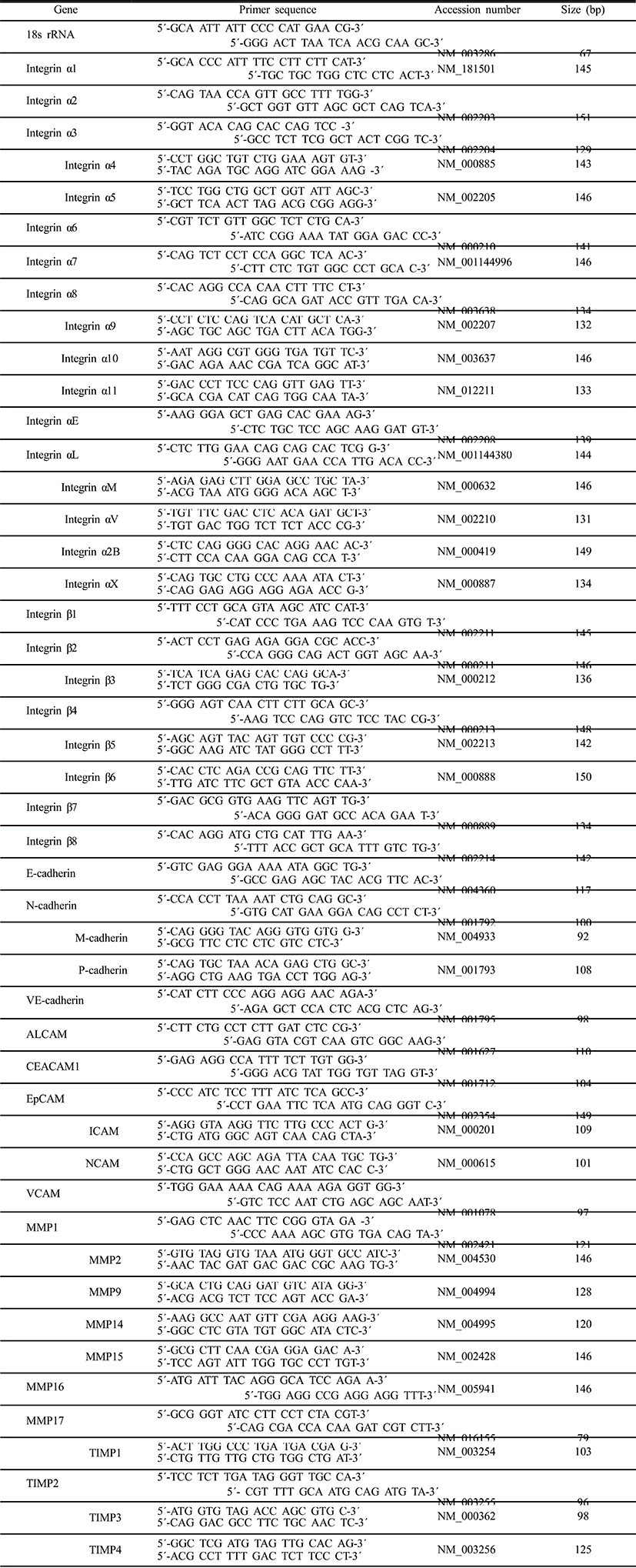
Total RNA was isolated using Tri-reagent (Ambion, Carlsbad, CA) according to the manufacturer’s instructions. RT-PCR was performed using a Techne PCR system TC-100 (Bibby Scientific, UK). Total RNA was reverse-transcribed using the following RT mixture: 20 mM MgCl2 (Bio Basic, Ontario, Canada), 10 × PCR buffer (BioBasic), 10 mM dNTPs mixture (BioBasic), 40 U/μl RNase inhibitor (BioBasic), 0.5 mg/ml random hexamer (Roche, New jersey, USA) and 200 U/μl AMV-RT (Invitrogen, Carlsbad, CA). Quantitative RT-PCR was performed using 150 ng/ml cDNA, primers, and 10 μl SYBRGreen Master mix (Roche, Mannheim, Germany), and 10 pmol of each primer using LightCycler 480 Real-Time System (Roche). Relative expression levels were normalized using 18s rRNA and the comparative CT (2–ΔΔCT) method. Primers used in the experiment are shown in supplementary Table 1.
Aggregations were collected with pipette tips, transferred into a 15-ml conical tube, washed twice with PBS, and fixed with 4% paraformaldehyde (Merck, Darmstadt, Germany) in PBS for overnight at 4°C and incubated at 4°C overnight in 200 μl of 20% sucrose solution in PBS. After incubation, aggregations were embedded in frozen section compound OCT (Leica biosystems, Richmond, USA). The mold sample was frozen by liquid nitrogen and stored at –80°C. Specimens were frozen-sectioned with 5-μm size using Leica CM3050S cryostat (Leica Microsystems) and processed for immunohistochemistry.
Aggregations were collected with pipette tips, transferred into a 15-ml conical tube, washed twice with PBS, and fixed with 4% paraformaldehyde in PBS overnight at 4°C. Then they were embedded in frozen section compound OCT (Leica biosystems, Richmond, USA) followed by freezing in liquid nitrogen. Specimens were frozen-sectioned with 5-μm size using Leica CM3050S cryostat and processed for immunohistochemistry. The sections were incubated with mouse monoclonal antibodies against integrin α2 (1:50; Santa Cruz, CA), rabbit monoclonal antibodies against integrin α2B (1:100; Abcam, Cambridge, MA) and mouse monoclonal antibodies against integrin αX (1:50; Santa Cruz) overnight at 4°C. They were then incubated with secondary antibodies: goat anti-rabbit IgG-FITC (1:200, Santa Cruz), goat anti-rabbit IgG-TRITC (1:200, Santa Cruz). Cell nuclei were stained with 4’-6’ diamidino-2-phenylindole (DAPI). They were visualized by confocal microscope (C1+, Nikon).
The protein samples were separated on 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE) gels and then transferred to nitrocellulose membranes (Millipore, Bedford, MA). The membranes were incubated with the mouse monoclonal antibodies against integrin α2 (1:100; Santa Cruz), integrin αX (1:100; Santa Cruz) and β actin (1:2,000; Santa Cruz) in blocking buffer (5% BSA in DPBS) overnight at 4°C. They were incubated in HRP-conjugated secondary goat anti-mouse IgG for 1 h at 37°C. The stained membranes were visualized by ECL kit (ATTO, Tokyo, Japan). Blotting experiments were repeated three times on every sample with similar results.
HEACs at p3-p6 were seeded on 12-well dish at a density of 7.3×104 cells/well. After 7 days of culture of HEACs, cells were treated with each 10 μg/ml of antibody against integrin α2, α2B or αX for 1 h at 37°C. Each antibody was added to DMEM containing 0.5% BSA and treated for 1 h. After that, cells of each well was washed with DMEM and cultivated in medium containing 10% HS. They were cultivated for 20 days.
Gelatinase activity of the conditioned medium was examined by gelatin zymography, which was performed as described previously (Park et al., 2005). Briefly, 10 μl of conditioned medium was mixed with the same volume of non-reducing sample buffer consisting of 500 mM Tris-HCl, 25% glycerol, 10% SDS, and 0.32% bromophenol blue (pH 6.8). Gelatin substrate for SDS-PAGE was prepared by adding 1 mg/ml bovine skin type B gelatin to the 8% resolving gel. After electrophoresis, gelatin-SDS gels were soaked with 2.5% Triton X 100 in 50 mM Tris-HCL buffer (pH 8.0) for 30 min and then incubated with incubation buffer (5 mM CaCl2, 0.02% NaN3, 50 mM Tris-HCl, pH 8.0) overnight at 37°C. At the end of the incubation, the gels were stained with Coomassie Brilliant Blue. All zymographic results were confirmed experiments three times.
RESULTS
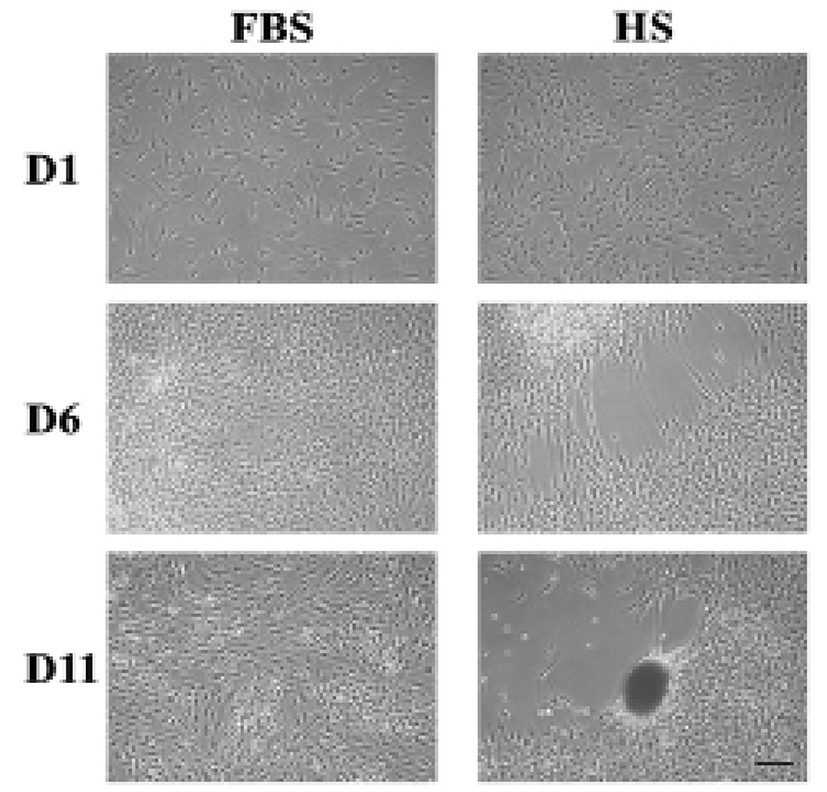
After around 6 days of culture of HEACs in a medium containing HS (HS medium), one or more cell-free area appeared in the surface of wells, and after 11 days, a population of cells nearby cell-free area became aggregated forming one or several cell clumps each well (Fig. 1). In many cases, morphology of the aggregates spheroid or ovoid but some were amorphous. In contrast, when cells were cultivated in FBS medium, HEACs did not show any morphological change until the end of culture. Interestingly, the formation of cell aggregation took place several times in a single spot, and proceeded very quickly such that cell migration and/or aggregation was hardly observed. The HS-induced aggregation was mostly observed between 9 and 15 days of culture.
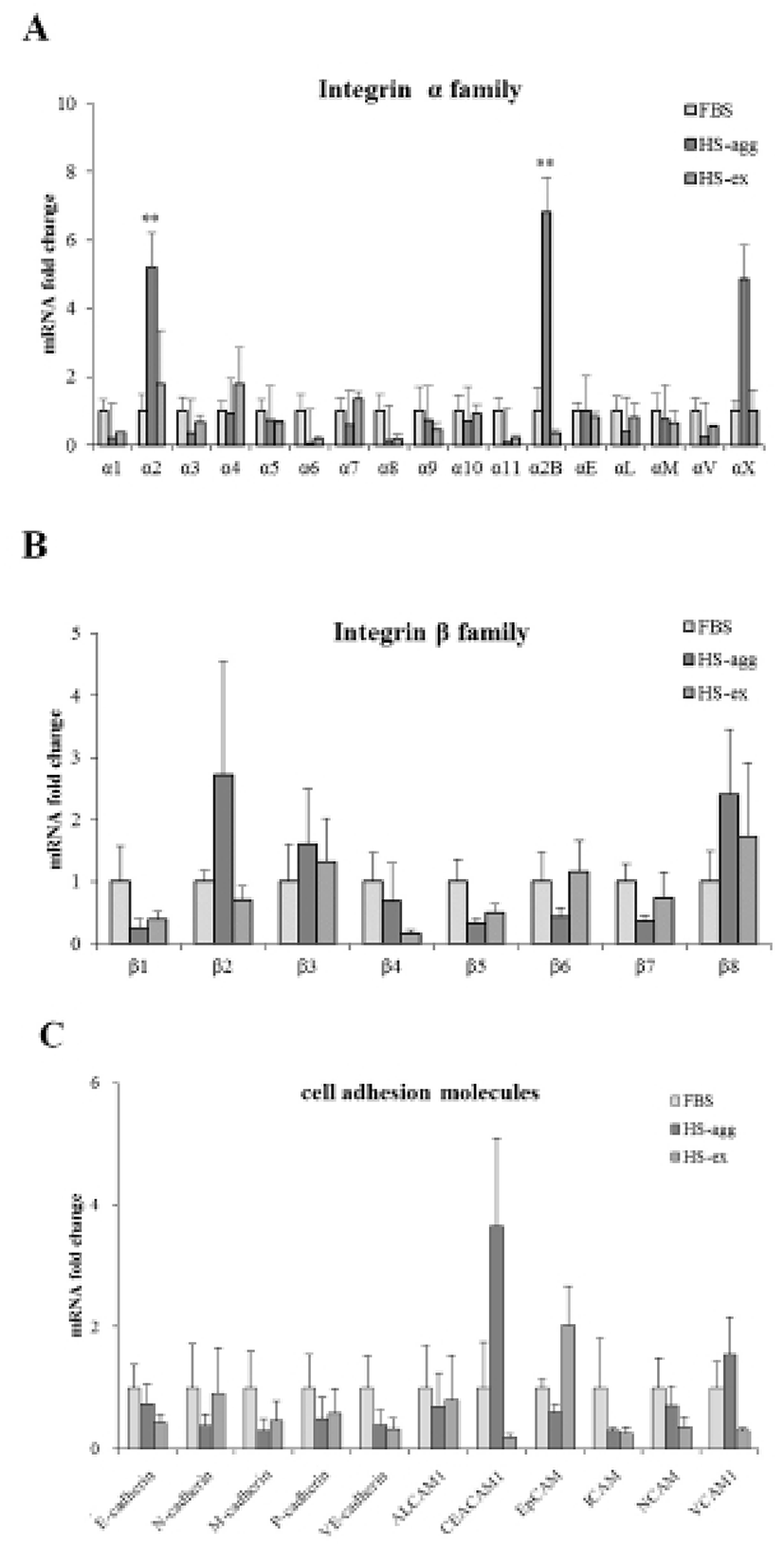
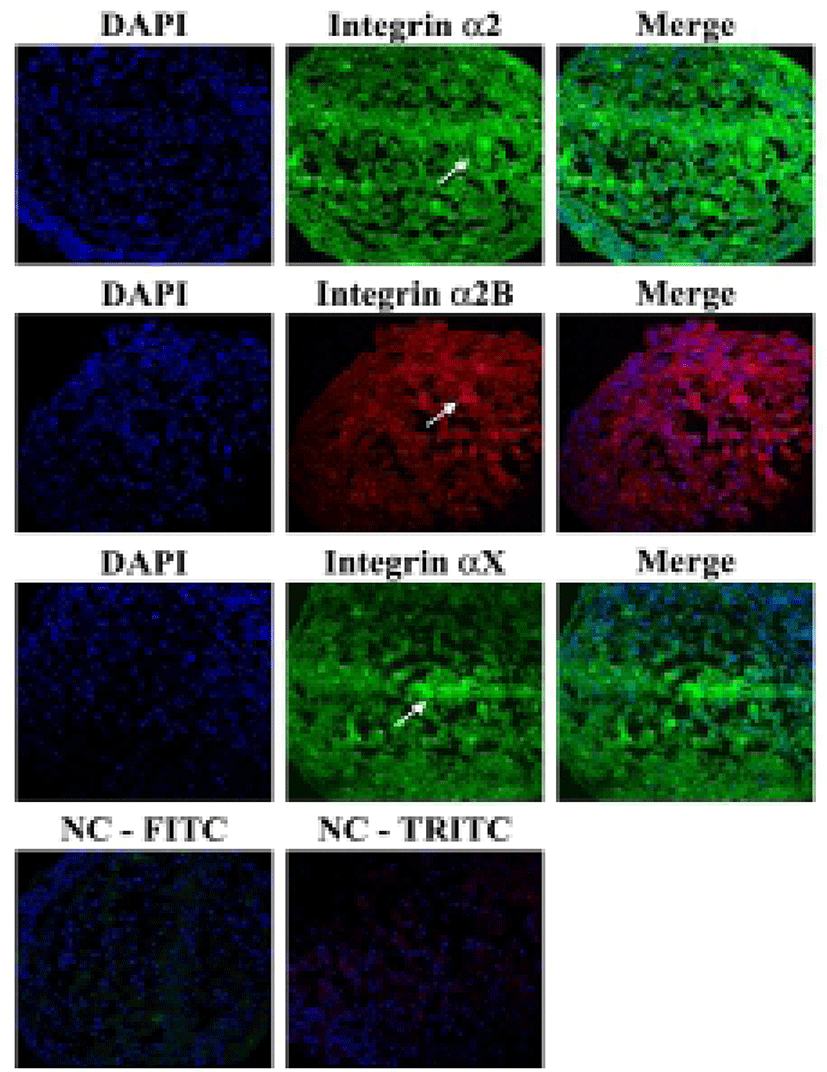
It was investigated whether the formation of aggregation was accompanied by the change of cell adhesion molecules. When cell aggregation was formed, both HS-agg and HS-ex were examined for the expression of the related genes. qRT-PCR analyses showed that among 17 members of integrin α family examined, expression of α2, α2B, and αX in HS-agg were greater by a factor of 5.2±1.8, 6.8±0.8, and 4.9±1.8, respectively, compared to the values of FBS-cultured cells. mRNA expression of the same genes in HS-ex did not show any significant change compared to the controls (Fig. 2A). Among the 8 members of integrin β family examined, β2 and β8 expression in HS-agg were slightly greater than those of control cells, but it was not significant. Interestingly β5 mRNA expression in HS-agg decreased. Again HS-ex did not show significant difference from that of the control (Fig. 2B). We have also examined mRNA expression of 11 cell adhesion molecules, and CEACAM1 mRNA of HS-agg showed 3.6±1.4-fold increased expression level compared to the FBS control while other mRNAs were not significantly different from the control (Fig. 2C). Immunocytochemical analyses demonstrated that integrin proteins of α2, α2B and αX were distinctly expressed in HS-agg of HEACs, while they were not detected in HS-ex or control FBS-cultured cells (Fig. 3).
Results of western blot analysis showed that staining intensity of α2 and αX proteins in HS-agg was stronger than that in HS-ex and FBS-cultured cells (Fig. 4A). After 7 days of culture in HS medium, cells were treated with each 10 μg/ml of antibodies against integrin α2, α2B or αX for 1 h, and then cultivated again in HS medium. Of the antibodies examined, integrin α2 antibody showed delayed aggregation by 4 days compared to the control HS-culture. Antibodies against α2B and αX did not show any delaying effect on the HS-induced aggregation (Fig. 4B).
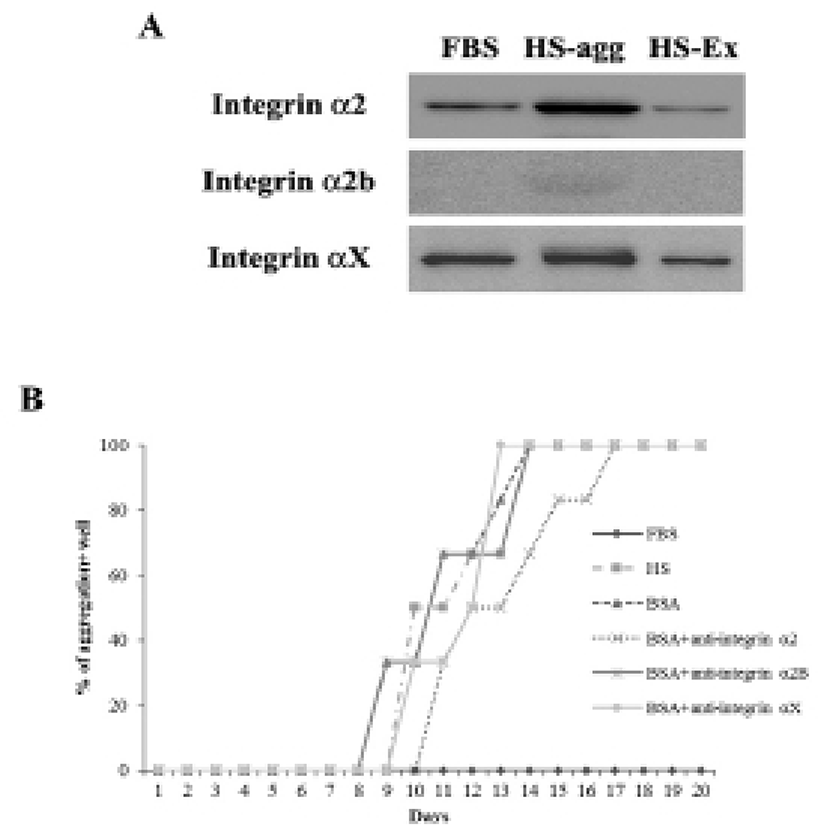
To examine if MMPs were involved in the aggregation, expression of various MMPs and membrane-type MMPs (MT-MMPs) were examined in HS-agg and HS-ex as well as in FBS-cultured cells by qRT-PCR (Fig. 5A). Among the 7 MMPs, MMP1 and MMP9 were strongly expressed in HS-agg. In case of MMP1, HS-agg showed 5.6±1.8-fold increased expression compared to the FBS-cultured cells. Moreover, HS-agg exhibited 9.2±0.8-fold increased MMP9 expression compared to the FBS-cultured cells. HS-ex did not show any increased expression than FBS-cultured cells. Other MMPs did not show significant changes of mRNA expression regardless of the HS treatment.
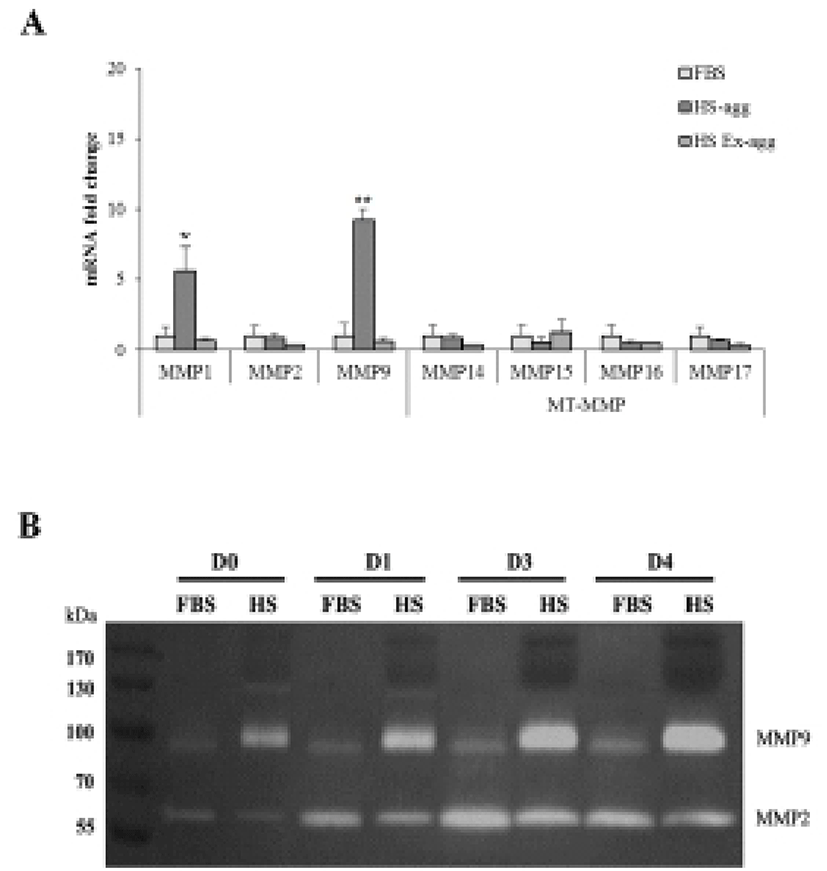
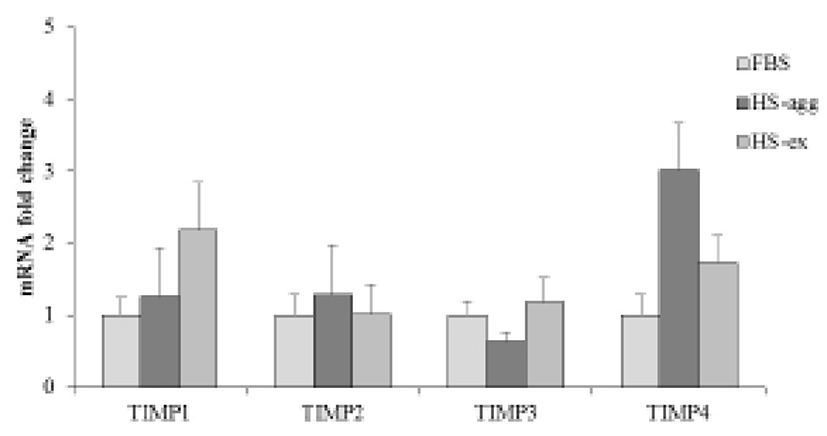
It was further examined by using gelatin zymography whether there was any changes of MMP activity in relation to the cell aggregation phenomenon. Conditioned media were obtained every day from cell cultures in FBS or HS medium during culture for 4 days. Strikingly HS-conditioned media exhibited a dramatic increase of MMP9 gelatinolytic activity beginning from 1 day culture (Fig. 5B). In contrast, conditioned media from cell cultures in FBS medium showed little MMP9 activity throughout 4 days. MMP2 activity of both FBS- and HS-conditioned media was discernable after culture for 1 day. While MMP2 activity of FBS-conditioned media showed a little increase thereafter, that of HS-conditioned media remained unchanged during 4 days. Both HS-agg and HS-ex showed a similar expression level of all four genes of TIMP-1, -2, -3 and -4 as compared to the FBS-cultured cells (Fig. 6). Although TIMP-4 mRNA expression appeared to be greater in HS-agg than other groups, it was not significantly different.
DISCUSSION
The highlight of this study is a finding that integrins α2, α2B, αX, CEACAM1, and MMP9 of HEACs undergo remarkable changes of expression when cells are aggregated by HS-culture. In mammals, eighteen α subunits and eight β subunits of integrins have been identified to date, from which 24 distinct heterodimer combinations have been observed (Prowse et al., 2011). Integrin ligation via cell-to-cell and cell-to-matrix contacts activates signaling pathways involved with differentiation, proliferation, and gene expression. Integrin α2 is combined with integrin β1 and functions as a signal transmitter for platelet activation (Jarvis et al., 2012). In addition to the increased expression of ITGA2 mRNA, treatment of its antibody showed delayed formation of aggregation in the present study, supporting the its role in migration during aggregation. Integrin α2B is also expressed in platelet, which forms heterodimer with integrin β3 (Neumann et al., 1998). A previous study has shown that the inhibition of the integrin α2Bβ3 receptor significantly impairs platelet recruitment to the growing thrombus. This observation suggests that platelet recruitment to venous stent thrombi occurs through the integrin α2Bβ3 receptor, at least in part (McBane et al., 2013). However, western blot results demonstrate that α2B protein expression was hardly observed in the present study. Integrin αX is combined with integrin β1 and interacts with iC3b and fibrinogen. It is expressed in leukocyte, which is involved with leukocyte emigration from the blood (Kaneider et al., 2010). Together with these results, our study suggests that integrins α2 and αX induced by HS culture might be involved in HS-induced aggregation during high-density culture. CEACAM1 was identified as possible mediators in HGF-induced keratinocyte migration and the functional relevance of CEACAM1 on epidermal cell motility was demonstrated using the HaCaT cell culture model (Schnickmann et al., 2009). In an implantation model of trophoblast spheroids on a monolayer of endometrial stromal cells, spheroid expansion was blunted by silencing of CEACAM1 (Gellersen et al., 2013). Inhibition of CEACAM1 with antibodies or soluble CEACAM1 or antisense oligonucleotides inhibited tubule formation of prostate cells grown in Matrigel by over 50% (Zhang et al., 2013). Results of these and our study together suggest an important role of CEACAM1 in cell migration.
Among seven members of MMP family examined, we found that mRNA expression of MMP1 and MMP9 genes specifically increased in HS-agg compared to the HS-Ex as well as control FBS-cultured cells. Also we observed an increased activity of MMP9 in conditioned medium obtained from HS-culture compared to the FBS-culture during successive 4 day-culture. In keratinocytes, cell migration is regulated by the catalytic activity of MMP1, and by anti-α2β1 blocking antibodies (Dumin et al., 2001). Using liver-injured mouse models, MMP-9 has been shown to contribute to the mobilization of BM cells in the injured liver by up-regulating the expression of CXCR4 on Lin(–) BM cells and attracting BM cells along its gradient of CXCL12 (Kawai et al., 2012). In a recent study wherein abdominal adipose-derived stem cells following short-term spheroid formation were used, enhanced in vitro migration was observed to associate with increased expression of MMP9 and -13 (Cheng et al., 2013). Upon transplantation in murine model with cutaneous wounds, these cells exhibited more cellular engraftment. While a number of studies have suggested a combinatorial role of MMPs and integrins during cell migration in vivo and in vitro, little report has demonstrated a relationship between MMP-9 and integrin α2, α2B and αX collagen receptor proteins. The present report, for the first time, suggest a possible role of MMP1 and MMP9 in migration and/or aggregation of human adult stem cells in vitro, via changes of integrins, the collagen receptor proteins.
HS has widely been studied by many researchers as a substitute of FBS in MSCs culture As well as pooled human AB serum, thrombin-activated platelet-rich plasma showed to provide a significantly higher proliferative effect on abdominal adipose tissue-derived MSCs (Kocaoemer et al., 2007; Bieback et al., 2010). These human supplements are better characterized regarding potential infectious threats, while providing a higher proliferation rate and retaining differentiation capacity and mesenchymal stem cell marker expression throughout long-term culture (Bieback et al., 2009). However, a higher proliferation effect of HS could not be a reason of cell aggregation as high-density alone cannot induce the aggregation as shown in our previous study (Song et al., 2012). Rather it seems due to a yet-unknown property of HS which is not present in FBS. It would be interesting to study whether the cell aggregation could be induced by other animal sera.
In summary, our results showed that the expression of integrin α2, α2B and αX in HS-agg increased during high-density culture in the presence of HS, and antibody against integrin α2 molecule delayed the aggregation. In addition, both MMP1 and MMP9 mRNA and MMP9 enzymatic activity also increased in HS-agg. Based on these results, it is suggested that integrins and MMPs might be involved in the HS-induced aggregation of HEACs.