INTRODUCTION
Sprouty (Spry) was first discovered as a common antagonist of fibroblast growth factor (FGF) and epidermal growth factor (EGF) signaling pathways in Drosophila (Hacohen et al., 1998; Kramer et al., 1999). In a search of the Expressed Sequence Tag database, three human homologs of the fly gene, designated hSpry1–3, were identified (Hacohen et al., 1998). The fourth mammalian homolog, hSpry4, was later discovered in mice and humans (De Maximy et al., 1999; Leeksma et al., 2002). Emerging evidence shows that Spry specifically inhibits mitogen-activated protein kinase (MAPK) signaling activated by a wide range of ligands, including FGF, platelet-derived growth factor (Gross et al., 2001), vascular endothelial growth factor (Impagnatiello et al., 2001), nerve growth factor (Wong et al., 2002), brain-derived neurotrophic factor (Gross et al., 2007), and glial cell line-derived neurotrophic factor (Ishida et al., 2007), but does not affect MAPK signaling activated by EGF (Sasaki et al., 2001). Spry2 reportedly potentiates biological effects induced by EGF via inhibiting EGF receptor degradation (Wong et al., 2001; Egan et al., 2002; Rubin et al., 2003). These findings suggest that Spry isoforms regulate MAPK signaling in a negative or positive manner, possibly depending on the subcellular context.
Autocrine FGF-induced extracellular signal-regulated kinase (ERK) 1/2 signaling occurs in embryonic stem cells (ESCs) and performs controversial functions to maintain the pluripotency of mouse ESCs (mESCs). It primes mESCs to initiate their differentiationin response to external differentiation-inducing stimuli as well as inducing the expression of inhibitors of FGF signaling to generate a negative-feedback loop that would facilitate reversion back to the original unprimed state (Kunath et al., 2007; Stavridis et al., 2007; Stavridis et al., 2010). ERK signaling can determine whether mesenchymal stem cells commit to the osteogenic orchondrogenic lineage. Inhibition of the ERK1/2 pathway completely abolishes chondrogenic differentiation and augments osteogenic commitment (Pelaez et al., 2012). Spry2 and Spry4 are the most highly expressed Spry isoforms in human ESCs. Knockdown of Spry2 decreases the proliferation and increases the death of human ESCs, whereas knockdown of Spry4 enhances the survival of the cells. This indicates that the Spry family regulates pathways involved in the proliferation and survival of ESCs (Felfly and Klein, 2013).
Although ERK signaling is a core signaling pathway to sustain the pluripotency of mESCs and to regulate the lineage commitment of mesenchymal stem cells, it is unknown whether Spry4 sustains the differentiation potential of mESCs and adult stem cells functions as it does in human ESCs. Here, we used mESCs as models to investigate the role of Spry4 in the stem cells. We report that Spry4 contributes to the maintenance of stemness and balanced differentiation of mESCs. When Spry4 was suppressed, stemness of mESCs was disrupted and the development of cartilage was facilitated interatomas formed by mESCs. Our results reveal that Spry4 is an important regulator of the stemness and differentiation of mESCs.
MATERIALS AND METHODS
J1 mESCs (cat #SCRC-1010) were purchased from ATCC (www.atcc.org) and maintained as described previously (Jung et al., 2012). To for membryoidbodies (EBs), mESCs were trypsinized to form a single-cell suspension and subsequently cultured on uncoated Petri dishes in ESC medium lacking leukemia inhibitory factor (LIF).
shRNA plasmids targeting mouse Spry4 were purchased (TL509780, Origene, MD, USA) to generate a stable Spry4- knockdown cell line. mESCs were grown to 80% confluence in 24-well plates, then transfected with shRNA plasmids using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Stable mESCs expressing shRNA for Spry4 were selected in 1 μg/mL of puromycin-containing culture medium. Knockdown of Spry4 expression was confirmed with real-time reverse transcription polymerase chain reaction (RT-PCR) analysis and immunoblot analysis.
Total RNA was extracted using TRIzol (Invitrogen), and 2–5 µg of total RNA was reverse-transcribed using the SuperScriptII™ First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. Real-time RT- PCR was performed using cDNA with the Quantitect SYBR Green PCR Kit (Qiagen, CA, USA). Reactions were performed in triplicate using an ExicyclerTM 96 real-time quantitative thermal block (Bioneer). For quantification, target genes were normalized against the glyceraldehyde 3-phosphate dehydrogenase gene.
Whole-cell extracts were prepared, and 20−50 μg of protein was resolved by SDS-PAGE and blotted using antibodies against Spry4 (ab59785, Abcam, MA, USA), phospho-Stat3 (Tyr-705; #9131, Cell Signaling Technology, Beverly, MA, USA), Stat3 (sc-482, Santa Cruz, CA, USA), cMyc (ab32072, Abcam), Nanog (ab80892, Abcam), Sox2 (sc-20088, Santa Cruz), phospho-ERK (M9692, Sigma), ERK2 (sc-154, Santa Cruz), and β-actin (sc-47778, Santa Cruz). Immunoreactivity was detected by enhanced chemiluminescence (Amersham, Buckinghamshire, England).
For the teratoma formation assay, cells were trypsinized, and 5 × 105 cells were suspended in a DMEM/Matrigel solution (BD Biosciences Inc., Franklin Lakes, NJ, USA) (1:1 ratio (v/v)). The cell/Matrigel suspension was injected subcutaneously into NOD/SCID mice (Charles River Laboratories, Yokohama, Japan). Teratoma formation was examined for 6 weeks after injection. The experiments were reviewed and approved by the Institutional Animal Care and Use Committee of CHA University. All procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Graphical data are presented as means ± SD. Each experiment was performed at least three times and subjected to statistical analysis. Statistical significance between two groups was determined using Student's t-test. A p value below 0.05 was considered significant. Statistical analysis was performed using the SAS statistical package v.9.13 (SAS Inc., Cary, NC).
RESULTS AND DISCUSSION
Activation of the FGF-ERK signaling pathway is required for naïve mESCs to initiate lineage differentiation by exiting their self-renewal status. Therefore, FGF signaling should be tightly regulated at multiple levels in ESCs. We previously reported that Spry1, a member of the Spry family of proteins, is necessary to balance the self-renewal and pluripotency of mESCs by inhibiting ERK1/2 signaling under self-renewal culture conditions (Jung et al., 2012). To increase our understanding of the regulatory mechanisms of ERK1/2 signaling in mESCs, we further investigated the role of Spry4 in mESCs.
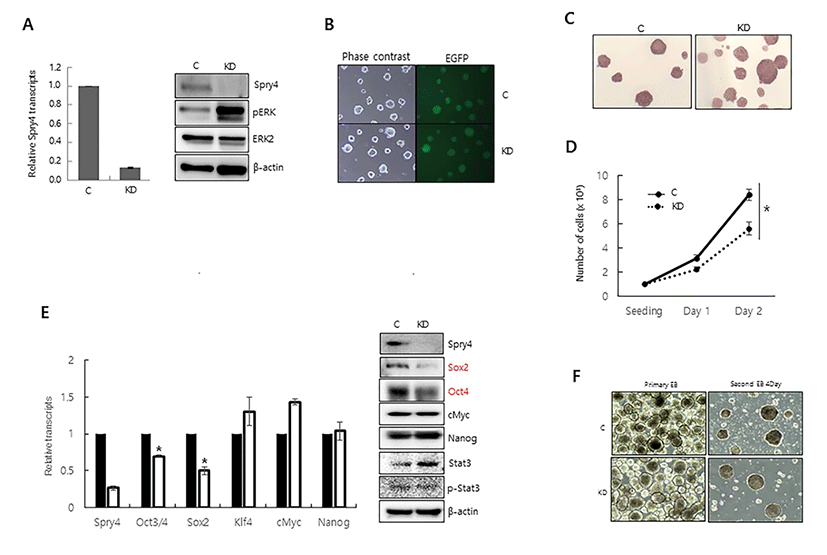
We first examined whether Spry4 modulates phosphorylation of ERK1/2 in mESCs. When Spry4 expression was suppressed by transfection of shRNA-targeting Spry4, ERK phosphorylation was significantly increased, suggesting that Spry4 suppresses ERK1/2 signalingin mESCs (Fig. 1A). We next engineered mESCs expressing green fluorescent protein and Spry4-targeting shRNA to generate a stable Spry4-knockdown mESC line (Spry4 KD), and then examined the effect of Spry4 suppression on the morphology and proliferation of these cells. Although Spry4 KD showed strong phosphorylation of ERK, it maintained the undifferentiated round shape of mESCs and alkaline phosphatase activity (Fig. 1B, 1C). However, the proliferation of Spry4 KD was lower than that of control cells (Fig. 1D). To examine whether altered Spry4 expression affects mESC self-renewal, we examined the expression of undifferentiated ESC markers. Expression of Oct4 and Sox2 was decreased at the mRNA and protein levels, whereas expression of c-Myc, Nanog, and phosphorylated Stat3 was not affected by suppression of Spry4 expression (Fig. 1E). We examined the ability of Spry4 KD to maintain an undifferentiated state by analyzing the efficiency of secondary EB formation (Felfly & Klein, 2013). The ability of Spry4 KD to maintain an undifferentiated state was com-parable to that of control mESCs, indicating that alteration of Spry4 did not affect the self-renewal of mESCs, despite the finding that suppression of Spry4 decreased cell pro-liferation and reduced expression of Sox2 and Oct4 (Fig. 1F). Our results are consistent with previous reports that Fgf4-null ESCs behave normally in the undifferentiated state in the presence of LIF, although they cannot exit the pluripotent state and initiate differentiation when LIF is removed (Kunath et al., 2007).
Autocrine FGF/ERK signaling is a pro-differentiation signal (Kunath et al., 2007); therefore, we hypothesized that the differentiation potential of Spry4 KD was higher than that of control mESCs. To test this hypothesis, we analyzed the impact of Spry4 suppression on the differrentiation of mESCs in xenografts. As expected, Spry4KD gave rise to larger teratomas than control mESCs (Fig. 2A). We next analyzed differentiation of the three germ lineages interatomas by hematoxylin-eosin (H&E) staining. The proportions of mesoderm tissues, including cartilage, bone, neuroglia and primitive mesoderm, as well as endoderm tissues, such as glands, were significantly increased in 6-week-old teratomas formed by Spry4KD (Fig. 2B, 2C). On the other hand, the proportion of immature cartilage was significantly lower in teratomas formed by Spry4 KD than in control teratomas, suggesting that the balanced differrentiation potential of mESCs was disturbed by suppression of Spry4. The expression level of Spry4 is much lower than that of Spry1 in mESCs (Jung et al., 1012). Despite it slow expression level, our results show that Spry4 plays a major role in control of the differentiation ability of mESCs.
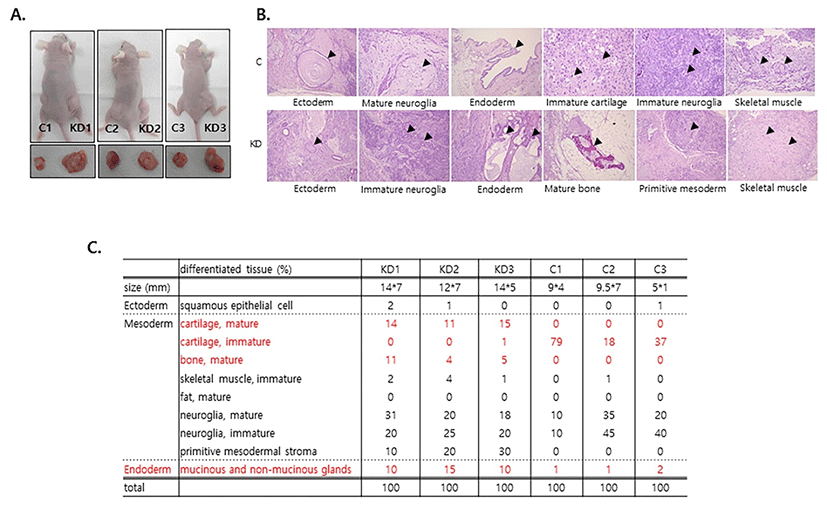
In our analysis of the lineage differentiation of teratomas formed by mESCs, we noticed that mature cartilage was highly developed in teratomas formed by Spry4 KD in comparison to control teratomas (Fig. 2C). Cartilage loss is a typical degenerative disease because cartilage has a limited regeneration potential; however, effective therapy remains elusive. Therefore, further work to investigate the detailed mechanism by which Spry4 enhances cartilage differentiation will contribute to enhance the differentiation efficiency of stem cells.

