INTRODUCTION
There are many examples of how environmental factors such as photoperiod and temperature, and social interactions such as aggression and meeting to the opposite sex, can alter reproductive status in sexually mature animals (Whittier et al., 1987; Wingfield et al., 1990; Hofmann & Fernald, 2000; Taranger et al., 2003; Stevenson et al., 2008; Maruska & Fernald 2011a). These signals are mainly integrated in the brain and conveyed to the gonads through the highly conserved brain-pituitary-gonadal axis. This common signaling pathway begins at the gonadotropin-releasing hormone (GnRH1)-containing neurons located in the preoptic/hypothalamic area of the brain, whose projections to the pituitary gland control the release of gonadotropins responsible for gonad growth and steroid hormone production (Maruska & Fernald 2011a; Jin et al., 2016).
The African cichlid fish, Astatotilapia burtoni, provides an interesting opportunity for analyzing neuronal changes because GnRH1 neurons in A. burtoni are remarkably plastic over short time scales in adult animals. GnRH1 neurons enlarge almost eight-fold and dramatically increase their dendritic arbors in the fish that ascend in dominance status from non-territorial and non-reproductive subordinate males (S), to territorial and dominant reproductive states (D) (Francis et al., 1993). During the first 4 days of social ascent, for instance, GnRH1 neurons grow to their D size, increase GnRH1 production, and extend their dendritic arbors. Since male reproductive competence can be switched on or off depending on external influence, i.e., social environment, and is strongly regulated by the plasticity in the GnRH1 neuronal system, GnRH1 neurons in the cichlid could be used as a model to test hypotheses about which structural genes are involved in the dramatic and reversible changes in GnRH1 neuron size. However, the mechanisms and molecules involved in mediating these morphological changes in GnRH1 neurons remain unknown.
Adult cichlid males can rapidly and reversibly switch between D and S states depending on the composition of the social environment, and such transformations produce a distinct pattern of behavioral and physiological changes (Maruska & Fernald 2013). When S males ascend in social status, they intensify their body coloration and increase dominance behaviors within minutes (Burmeister et al., 2005). Although they also described that even D males apparently began their typical aggressive behaviors anew each day, these data were sampled only during the 20 minutes following the first dominance behaviors because the newly ascended males were then sacrificed. As far as we know, little is known about how D behaviors change during days of social transition.
Target of rapamycin (TOR) was originally identified in yeast and later characterized in mammals (mTOR), structurally and functionally conserved mammalian counterpart (Schmelzle & Hall, 2000). mTOR controls neuronal soma size, local protein synthesis in dendrites, axon growth and navigation, and synaptic plasticity (Kwon et al., 2003), as well as organization of the actin cytoskeleton, membrane traffic and protein degradation, protein kinase C signaling, ribosome biogenesis, and transcription (Schmelzle & Hall, 2000). TOR is hypothesized to serve a conserved role in the control of cell growth (e.g., central controller of cell growth) and synaptic plasticity (Schmelzle & Hall, 2000), but its function in non-mammalian vertebrates is relatively unclear. In addition, whether TOR controls neuron size in neuroendocrine cells like GnRH neurons remains unknown.
In the present study, we have microinjected rapamycin into the third ventricle of D male brains to understand how TOR inhibition affects the behavior of the animals. Furthermore, since rapamycin influences cytoskeletal rearrangement (Laplante & Sabatini, 2012), we examined GnRH1 neuron size in the brain and germ cells in the testicular seminiferous tubules.
MATERIALS AND METHODS
Cichlid fish A. burtoni (N=50; mixed sexes) purchased from AquaLab Co. (Daegu, Korea) were maintained in semirecirculating aquaria (60 × 30 × 40 cm) under environmental conditions (25~27°C; constant aeration; 12h light: 12h dark cycle with full spectrum illumination by LED lighting (6,500 lux, Paisiz, Korea), and fed ornamental fish pellets (Tetrabits+25%, Fish Aquarium Home, India) twice a day. Aquaria contained corallite-covered bottoms with half terra cotta pots that served as spawning territories. All experimental fish were handled in accordance with the guideline, procedures and protocols of care and use of laboratory animals, Stanford Administrative Panel for Laboratory Animal Care.
Adult D and S males (each one individual, body weight 5.2~5.7 g, body length 5.0~6.0 cm) were placed in the half side of fish tank separated by a black acrylic barrier with two adult females (body length 4.0~5.0 cm) (Fig. 1). This compartment was isolated from each community group so that a group could not interact physically to the other one. At least two days ago, two community groups of fish were prepared for behavior monitoring. There are nine wellclassified behaviors in this cichlid fish species (Fernald, 1977). We have chosen three reproductive behaviors, i.e., chasing, courtship display and spawning site entry. Briefly, chasings define rapid swims of D male directed towards another female fish. Courtship displays were defined as rapid quivers by the male with presentation of the anal fin egg spots, and spawning site entries as the male entered half terra cotta pots as a spawning shelter (Maruska & Fernald, 2013).
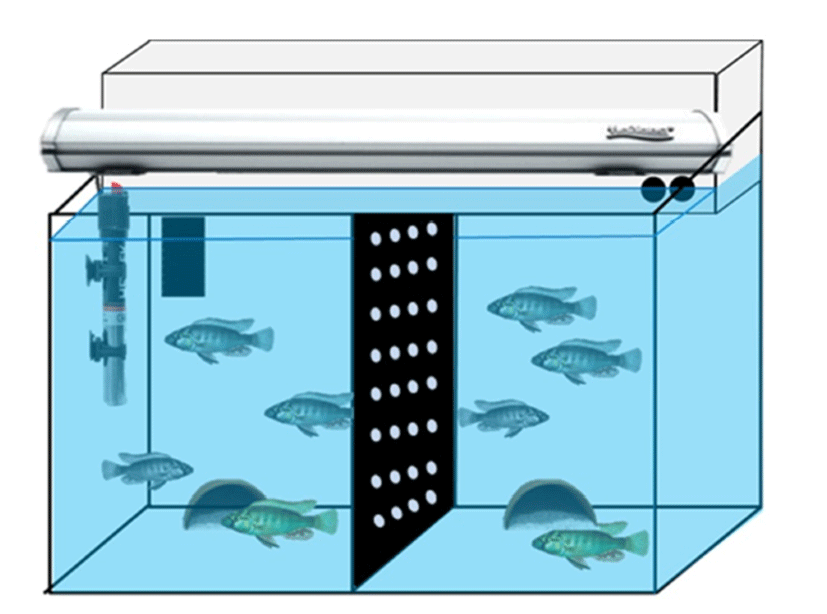
Experimental procedures for the intraventricular injection were generally performed according to a previous report for Nile tilapia Oreochromis niloticus (Ogawa et al., 2006). Briefly, fish were anesthetized in 0.01% solution of 3- aminobenzoate methanesulfonate (MS-222; Omnilab, Bremen, Germany) and were then made a puncture on the scales and scull on top of the brain using a 20G needle. A 2 μL Hamilton syringe (Hamilton, Nevada, USA) was stereotaxically injected into the third ventricle (stereotaxic X, Y, Z coordinates for preoptic-GnRH1; 0.0/+2.5/+6.0 mm) for each individual. Rapamycin (LC Labs, Woburn, MA, USA) was diluted by dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) with methylene blue solution (Daejung Chem., Siheung, Korea). Final concentration of rapamycin solution was set to 20 μg/μL in 4% methylene blue solution and 1.0 μL was microinjected into third ventricle. In the control fish, the same volume of vehicle solution was injected into the brain. Rapamycin- and DMSO-injected groups (n=3 each group) were monitored for further two days in the fish tanks. Behaviors were recorded for 20 min for later quantification beginning at light onset (08:00 AM) using a digital video camera (FDR-AXP35, Sony, Japan) on the day before intraventricular injection and days 1–2 post-injection. Behavioral counts by video recordings were done without individual identification, i.e., rapamycin- and vehicle- injected fish.
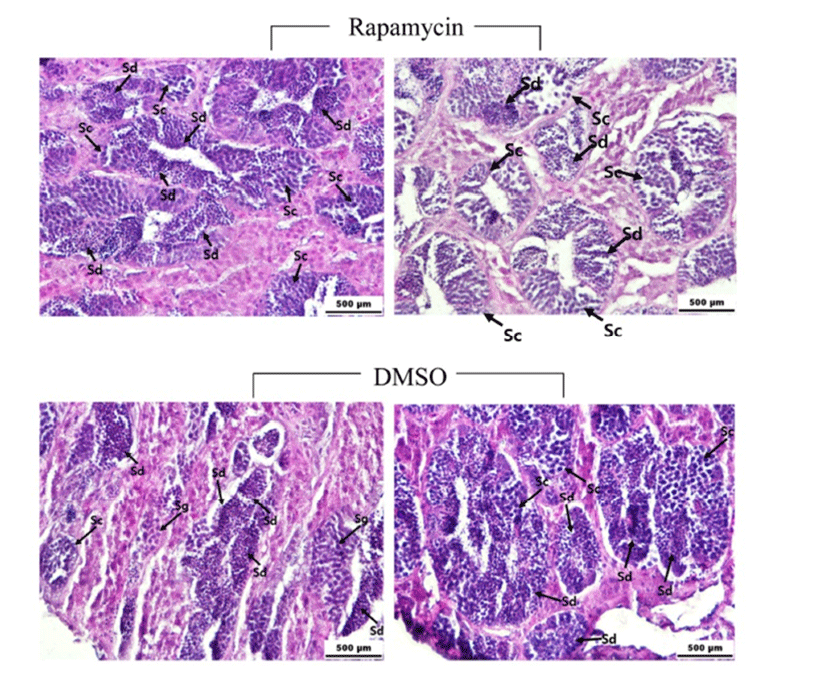
Testes and brains were sampled from the rapamycinand DMSO-injected fish after final video recordings. Total body weight was obtained for each specimen examined followed by the removal and weighing of the testes to calculate GSI (gonad weight × 100/body weight). Brains were removed following rapid cervical transection and testes were then fixed in 4% paraformaldehyde solution (Wako Pure Chem., Osaka, Japan) for histological examinations. Fragments of fixed brains and testes were embedded in paraffin and sectioned at a thickness of 7 μm. Serial sections for testes were affixed to glass slides, underwent clearing, hydration and finally stained with hematoxylin and eosin solution. Processed slides were examined using a light microscope (Nikon Eclipse E200, Nikon, Tokyo, Japan). For immunohistochemical observation, brain sections were rinsed in deionized water and phosphate buffered saline (PBS) three times. Nonspecific binding was blocked with a 2 h incubation in PBS supplemented with 0.2% bovine serum albumin (BSA), 10% normal goat serum (Invitrogen, Grand Island, NY), and 0.3% Triton X-100. GnRH1 neurons were detected with a polyclonal guinea pig antibody for cichlid GnRH1 (Ma et al., 2015). Slides were incubated with the anti-GnRH1 antibody diluted 1:1,000 in PBS including 0.3% Triton X-100 at 4°C. Following primary antibody incubation, brain slides were washed three times for 5 min in PBS and incubated for 2 h at RT with a goat anti-guinea pig IgG HRP (Santa Cruz Biotech., Dallas, TX, USA) diluted 1:500 in PBS. After rinsing in PBS including 0.3% Triton X-100 three times, slides were treated with DAB-peroxidase substrate solution (1% DAB, 0.015% H2O2, 0.01 M PBS, pH 7.2) for 10 min. Slides were then washed three times for 5 min in PBS and cover-slipped with a mounting medium.
RESULTS
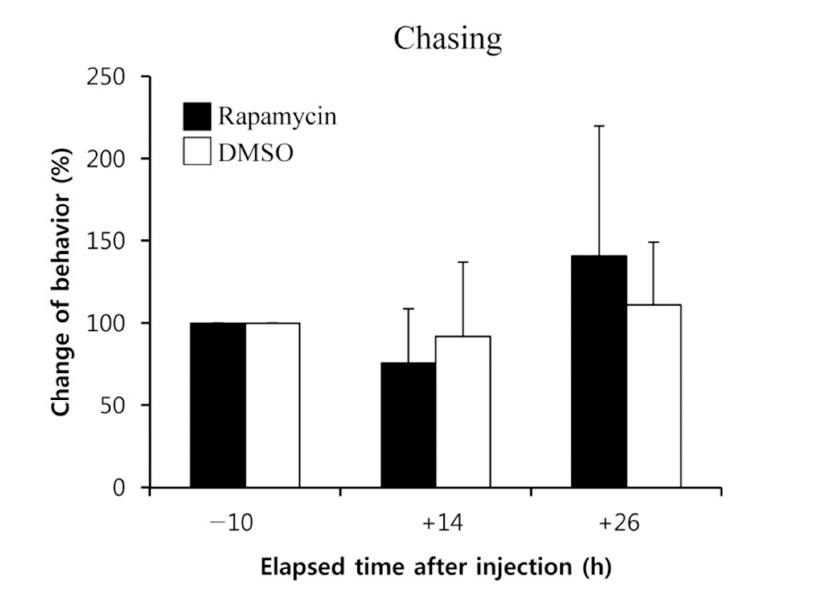
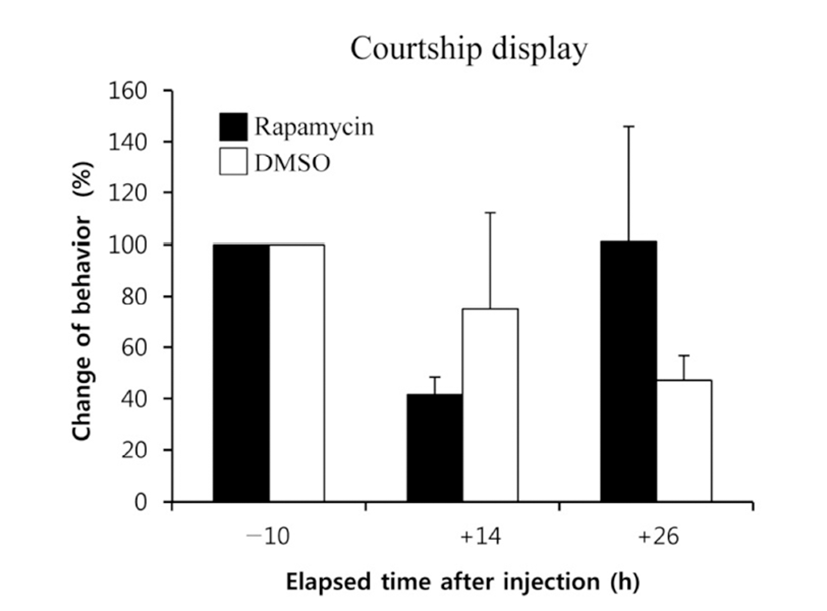
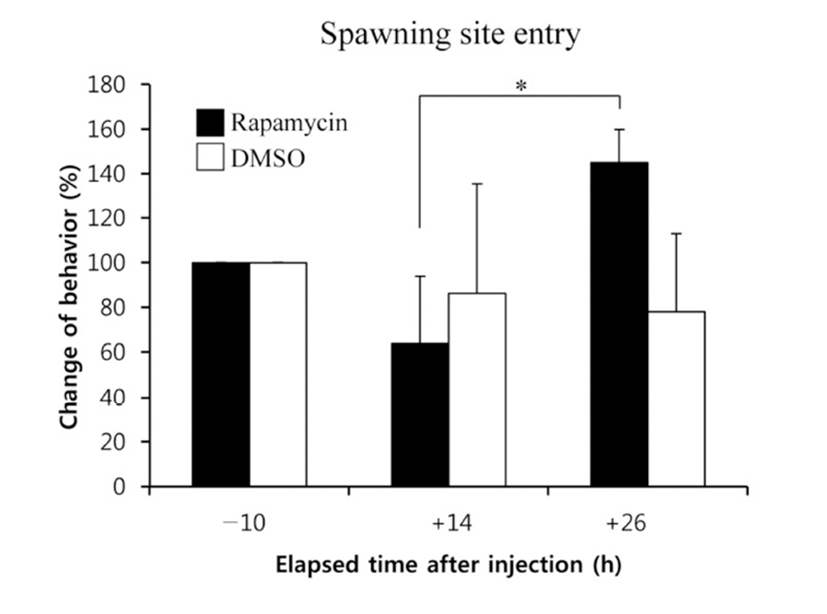
When the lights come on in the examination tank, subjects show a rapid and steady increase in rates of dominance behaviors, such as chasing, courtship displays, and spawning site entries, that typically reached 5~10 behaviors/min after light onset (Maruska & Fernald, 2010). In the present study, we also observed the typical behaviors, suggesting that the D males displayed a normal range of intrinsic performance. Chasing behaviors in rapamycin-injected fish did not show significant changes compared to that of DMSOinjected fish by 26 h post-injection (Fig. 2). Courtship display in rapamycin-injected fish slightly decreased at 14 h but increased at 26 h post-injection when it was compared to that of control fish, although the differences were not significant (Fig. 3). Behavior rates of spawning site entry increased in rapamycin-injected fish at 26 h post-injection than at 14 h post-injection significantly (P<0.05), whereas the DMSO-injected control fish did not show any change during experimental examination (Fig. 4).
We examined the GnRH1 neurons in the POA to judge whether the behaviors of the rapamycin-injected fish are dependent on the neuronal activities. The soma sizes of GnRH1 neurons in fish injected with rapamycin showed a tendency of decreasing compared to those of DMSOinjected fish (Fig. 5).

There was no significant difference of GSI between the rapamycin- and DMSO-injected fish (0.69~0.95). Histological observations showed that the majority of the germ cells in the testes of rapamycin- and DMSO-injected males are spermatocytes and spermatids (Fig. 6). However, the most advanced spermatogenetic cells, e.g., mature spermatozoa, were not detected in either rapamycin- or DMSO-injected fish.
DISCUSSION
A. burtoni has become a valuable vertebrate model species for studying social environment influences on behavior, the brain, and reproductive plasticity (Fernald, 1977; Maruska & Fernald, 2010, 2013). The males of this species exist in two distinct phenotypes, non-reproductive subordinate S and dominant reproductive D types. The D males of yellow and blue coloration have territories that they defend strongly and spend considerable time attempting to court with matured females, whereas the S males without territories school with females and other S males and flee from aggressive D males (Fernald, 1977; Maruska & Fernald, 2010). Although these social behaviors are evidently linked to hypothalamic neurons expressing GnRH1, pituitary gonadotrophic and testicular activities, (Maruska & Fernald, 2011b), the detailed mechanisms and upstream molecules involved in mediating these social behaviors are largely unknown. In the present study, TOR inhibitor rapamycin was monitored as a candidate responsible for sexual behavior and GnRH1 neuron enlargement in male A. burtoni.
Our data show that intraventricular injection of rapamycin has only a marginal impact on the social and reproductive behaviors of the D males. As mentioned by Maruska and Fernald (2010), three distinctive male behaviors, chasing (51~165 times/20 min), courtship display (15~86), and spawning site entry (11~33), were continuously shown in the aggressive D males during this experiment. Although spawning site entry slightly increased in rapamycin-injected fish at 26 h post-injection, other behaviors did not show significant changes between experimental and control groups. In male mice, inhibition of hypothalamic mTOR signaling pathway by rapamycin regulates sympathetic nerve traffic and behavioral rhythms (Muta et al., 2015), even though mTOR generally operates as sensor of cellular energy status and effector for its coupling to cell growth and proliferation (Schmelzle & Hall, 2000). Since the final concentration of rapamycin solution (20 μg/μL) injected into brains is not lower compared to other experiments in rats (50 μg/μL) when the body mass is considered (Hebert et al., 2014), it is likely that D males’ dominant behaviors would not be affected by TOR inhibitor within a short-term period.
Increased soma size of GnRH1 neurons in D males than that of S males provides an important clue to understand the social and reproductive behaviors in this species (Francis et al., 1993). Although the activity of GnRH1 neurons is crucial for the pituitary and gonadal regulatory mechanisms, the regulators of GnRH1 neuron activities are still poorly understood in non-mammalian species (Karigo & Oka, 2013). Some of earlier experiments using neuropeptides and neurotransmitters such as neuropeptide Y, noradrenaline, serotonin, and dopamine demonstrated their activities on GnRH1 neurons by measuring the amount of GnRH1 peptide released in fish species both in vitro and in vivo examination (Zohar et al., 2010). Further, in primate pituitary rapamycin treatment abolished a stimulatory effect of kisspeptin on gonadotropin secretion (Luque et al., 2011), suggesting that the stimulation of pituitary gonadotropin elicited by kisspeptin is mediated by mTOR. In the present study, we suggested that the mTOR signaling cascade may mediate GnRH1 neuronal activity in cichlid D males because a tendency of decreasing soma size of GnRH1 neurons was shown in D males received an intraventricular injection of rapamycin. To determine whether the mTOR signal pathway is closely linked to GnRH1 neuron activity, a series of fine-tuned in vivo and in vitro experiments should be performed. The developmental stages of testes did not show marked changes between rapamycin- and vehicle-injected fish. The majority of the seminiferous tubules in both groups’ testes were filled with spermatocytes and spermatids, suggesting that the behaviors of D males, i.e., chasing, courtship display, and spawning site entry, are not likely associated with actual spermiation. With regard to aggressive behaviors of reproductively active cichlid and zebrafish males, testosterone secretion was considered as a promising trigger of the characteristics (Maruska & Fernald, 2010; Teles & Oliveira, 2016). Thus, testosterone level could be a good indicator for mTOR-mediated reproductive activities and behaviors in D cichlid males.

