INTRODUCTION
Gonadotropins are glycoprotein hormones having a stimulating effect on the gonads. Among them, LH and FSH are secreted by the anterior pituitary gland of all vertebrates and some invertebrates; chorionic gonadotropins(CG) are uniquely secreted by placenta of primate and equine species (Pierce & Parsons, 1981; Choi & Smitz, 2014). Gonadotropin and thyroid-stimulating hormone (TSH) are heterodimers consisting of two peptide chains, an alpha (Cgα) chain and a beta (β) chain; the α subunits of these four glycoprotein hormones are identical; however, their β chains are unique and confer biological specificity. (Fan & Hendrickson, 2005).
The cDNAs of the gonadotropin subunits, which are encoded by a single gene, have been cloned from the pituitaries of human, mouse, and rat (Fiddes & Goodman, 1981; Chin et al., 1981; Chin et al., 1983; Godine et al., 1982; Talmadge et al., 1983; Gharib et al., 1989; Kumar & Matzuk, 1995). Interestingly, presence of more complicated transcripts of LH-β subunit gene in rat testis was accidently found when the testis was used as negative control (Zhang et al., 1995a). The authors also demonstrated that expression of testicular LH-β subunit was confined in seminiferous tubules to spermatids, and the translated products were also localized in the cytoplasm of elongated spermatids (Zhang et al., 1995a).
We hypothesized that mouse testis also has potential to produce the tissue specific LH-β subunit with similar structure (i.e. cDNA sequences) to the rat testicular forms. To verify our hypothesis, we examined whether the transcripts for LH subunits and their translated products were existed in the mouse testis using RT-PCR and specific immunohistochemical analyses.
Materials and Methods
Adult male ICR mouse (about 5 months old) were obtained from Han-Lim Animal (Gyunggi-do, Korea) and reared in Sangmyung university animal facility under conditions of 12-h light/dark cycle (lights on at 07:00 h) and constant temperature of 22±1℃. Mice had free access to normal chow and tap water ad libitum. After allowing 7 days acclimation, mice were sacrificed and the testes and pituitaries were collected for RT-PCRs and immunohistochemistry. All procedures used were approved by the Animal Care and Use Committee (2013-01-03) at Sangmyung University.
Total RNAs were isolated from tissue samples using TRIzol Reagent (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The extracted RNAs were used in RT-PCR reactions carried out with Maxime™ RT PreMix (iNtRON, Korea) and AccuPower PCR Premix (Bioneer, Korea) according to the manufacturer’s instructions. Primer sequences and the specific PCR conditions used in this study were listed in Tables 1 and 2, respectively. The reaction products were analyzed by gel electrophoresis in 1.5% agarose gel (75 V, 65 min) and visualized by ethidium bromide staining. The band intensities were measured using the image analysis system (ImagerⅢ-1D main software, Bioneer, Korea). The testicular LH-β PCR products were used for further analysis of cDNA structure. Sequence analyses were done by a commercial sequencing service company (Macrogen, Korea).
The primary antibodies against LH subunits used in this study were obtained from NIDDK-NHPP program as a courtesy of Dr. A. F. Parlow; rabbit polyclonal antibodies against mouse LH-β subunit (diluted 1:500) and mouse Cgα subunit (diluted 1:250). Tissue specimens were fixed in 4% paraformaldehyde solution, and the fixed tissues were serially dehydrated in graded ethanol and xylene. Specimens were embedded in paraffin block, and the tissues blocks were cut at 6um using microtome (HM350S, MICROM, Germany). After being dewaxed in xylene and hydrated in ethanol to 10mM sodium phosphate buffered saline (PBS, pH 7.2), the sections were subsequently incubated overnight at room temperature with primary antibodies. The sections were incubated with second antibody, goat anti-rabbit IgG conjugated with horseradish peroxidase (Santa Cruz Biotechnology, USA; diluted 1:200), for 2 hr. Finally, positive signals were detected by Peroxidase Polymer Anti-Rabbit IgG Kit (Vector Laboratories, USA; diluted 1:50).
Results
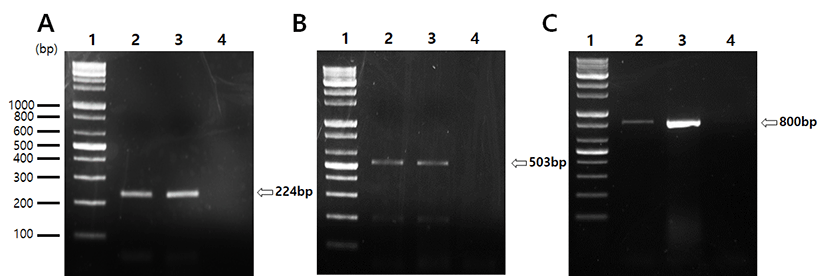
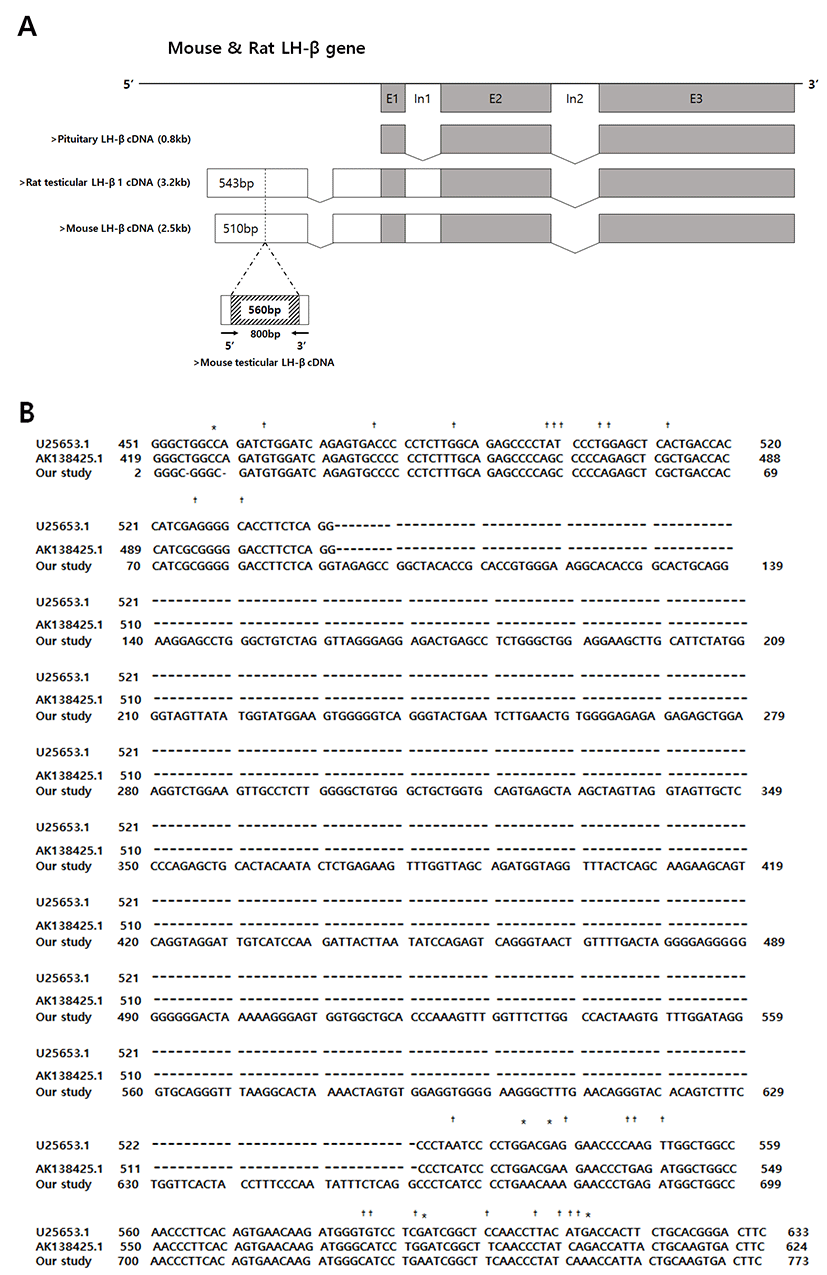
To determine whether the mRNAs for both Cgα subunit and LH-β subunit (specifically pituitary or testis type) are present, total RNAs isolated from pituitary (as positive control) and testis of normal adult mice were subjected to RT-PCR analyses. The amplifications revealed the presence of the identical products in all three reactions. The expected product sizes for mouse Cgα and LH-β known as pituitary type were 224 bp and 503 bp, respectively (Fig. 1A & 1B). The testicular type of LH-β products were produced by a primer set based on the rat sequences, with unexpected size of 800 bp (Fig. 1C). Sequence analysis of this products revealed that the proximal and distal parts (2-82 and 661-773 bp, respectively) were homologous to the rat testicular LH-β cDNA, and the relatively long middle part (83-660 bp) was a unique mouse-specific region (Fig. 2A & 2B).
Immunostaining using anti-Cgα polyclonal antibody was performed on the testis tissue sections from normal adult mice (Fig. 3B). Cgα positive signals, though weaker than LH-β signals, were in the round spermatids, elongated spermatids and mature sperms. The results also showed moderate staining in the cytoplasm of the spermatocytes.
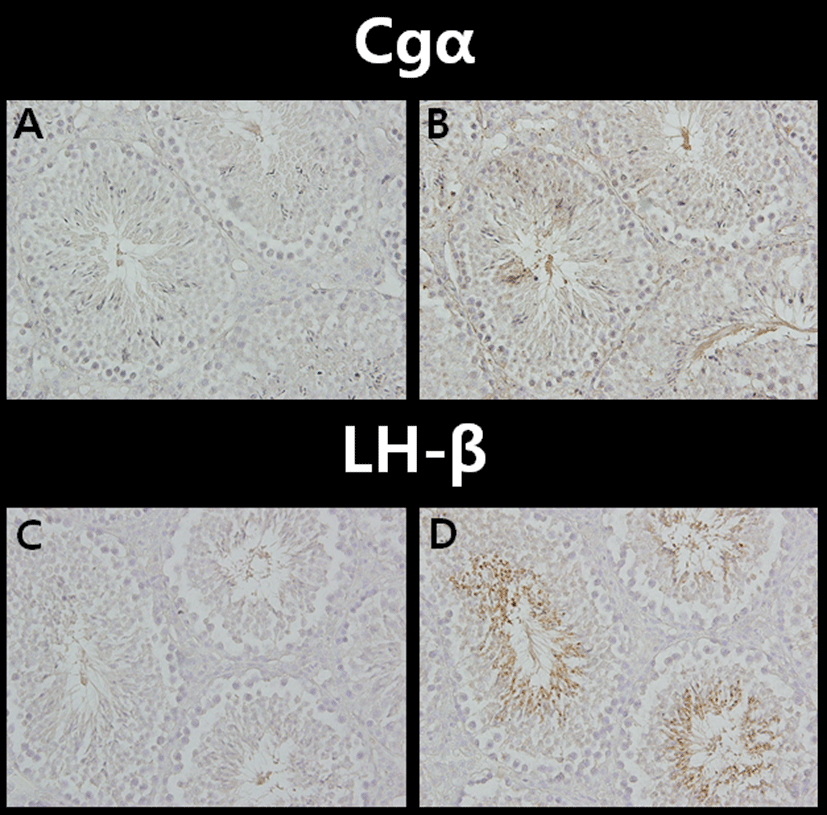
Immunohistochemical analysis of LH-β revealed that the more intense positive signals were present in round spermatids, elongated spermatids and mature sperms (Fig. 3D). The negative controls were treated in the same manner as the other samples except PBS was used instead of the primary antibodies against Cgα or LH-β (Fig. 3A & 3C).
Discussion
In the present study, we demonstrated for the first time the presence of the transcripts for Cgα and LH-β in mice testis, and the translated products are localized in the round and elongated spermatids and mature sperms. These findings are in good agreement with the results of the previous study on the rat testicular LH expression (Zhang et al., 1995b). Furthermore, we provided evidence on Cgα immunostaining which was omitted in the previous study. The localizations of immunoreactive Cgα and LH-β in mice testis roughly overlapped, and the staining intensities varied; LH-β signals were stronger than that of Cgα. Particularly, our sequence analysis revealed that the transcripts for mice testicular LH-β have unique structure, containing additional part which was not found in the rat testicular transcripts. Whether this unique product is oriented from intron or exon is interesting question. We speculate this could be a part of novel exon, since the product is lacking the typical end dinucletides of most introns (5'-GT and AG-3'). And if genomic DNAs were contaminated in our PCR samples, the LH-β PCR for amplification of pituitary type product should also exerted bigger products containing two intron-derived insertions. If the unique product is oriented from intron, the published structure of the rat testicular LH-β transcripts (known as TLHβ 1and 2) in the previous study should be corrected (Zhang et al., 1995b).
Extrapituitary production of LH has been found in many tissues (Harvey et al., 2012). LH is found in the fetal rat brain and persists in the adult rat and human (Hojvat et al., 1982; Bowen et al., 2002), in the pineal gland (Noteborn et al., 1992). The presence of LH receptors in the hippocampus (Lathe, 2001) and cultured neonatal rat brain glial cells (Al-Hader et al., 1997), suggests that brain LH may stimulate this region and possibly influence synaptic function, and may act as a neuromodulator of reproductive behavior. The local LH production in the brain as well as its presence in the peripheral tissues is thought to explain the persisted LH level in the circulation of hypophysectomized rats (Shin et al., 1986; Harvey et al., 2012). Moreover, LH is produced by lymphocytes in blood, and this may act as a cytokine-like determinant of T-cell proliferation (Hotakainen et al., 2000; Sabharwal et al., 1992). And finally, LH is also present in the reproductive organs such as gonads (Zhang et al., 1995a & b; So et al., 2005) and placenta (Al-Timini & Fox, 1986), though the its physiological role is unclear. Unlike authentic pituitary LH, serum concentration of the extrapituitary LH is probably too low to induce the endocrine function such as triggering ovulation and stimulating steroidogenesis. Instead, local autocrine or paracrine roles such as fine tuning of target tissue physiology could be suggested. Regarding the mouse testicular LH, one can also postulate its plausible roles by its specific localizations; in the regulation of spermiogenesis and/or capacitation during transport through male and female reproductive tracts.
In conclusion, the present study provides evidence for the presence and localization of the LH subunits in mouse testis. Our ongoing studies on the structures of LH-β transcripts and their testis-specific promoter will be useful for precise understanding of local regulation of this gene and preparation of the loss-of-function animal model through conditional knock-out technique.

