INTRODUCTION
A significant component of the reinforcing properties of ethanol may be its pronounced anxiolytic effect, and individuals with clinically diagnosed anxiety disorders are at greatly increased risk for ethanol abuse and dependence. The amygdala formation has a critical role in the action of anxiolytic drugs such as ethanol, and is likely to be an important brain area for investigating the mechanisms of regulation of anxiety by ethanol.
In the basolateral amygdala (BLA), ethanol inhibits projection neuron activity in vivo (Perra et al., 2008). In the BLA in vitro preparation, ethanol increases spontaneous and evoked GABA release (Zhu & Lovinger 2006; Silberman et al., 2008), inhibits glutamatergic transmission (Gean 1992; Calton et al., 1998; Lack et al., 2008) and inhibits voltage-dependent calcium channels (Calton et al., 1999). However, studies of ethanol effects on recurring neuronal circuit activity in brain areas such as the amygdala are rare. In the BLA, compound postsynaptic potentials (cPSPs) represent spontaneous circuit activity that requires both GABA and glutamate neurotransmission (Smith & Dudek, 1996; Rainnie, 1999). It was recently demonstrated that the frequency of cPSPs recorded in vitro in BLA is modulated in a consistent fashion by neuropeptides known to regulate anxiety states (Chung & Moore, 2009). Because ethanol also modulates glutamate and GABA neurotransmission, whether cPSPs might be modulated by ethanol was investigated using a whole cell patch recording technique in a rat brain slice preparation containing the amygdala formation. Using cPSPs as a model, it was attempted to characterize ethanol effects on neuronal circuit activity in BLA. Since ethanol is known to have age-dependent effects on anxiety-related behaviors in rodents (Silveri & Spear, 1998; Varlinskaya and Spear, 2002a, 2002b), effects of ethanol on cPSPs recorded from slices from immature and mature rats was also investigated.
MATERIALS AND METHODS
All procedures were approved by the Duke University and Durham VAMC Animal Care and Use Committees. Male Sprague-Dawley rats (16 to 23 or 75 to 79 days old) were decapitated under isoflurane anesthesia. Brains were quickly removed and placed into a chilled solution containing (in mM) 2.5 KCl, 1.25 NaH2PO4, 10.0 MgSO4, 0.5 CaCl2, 26.0 NaHCO3, 11.0 glucose, and 234.0 sucrose bubbled with 95% O2/5% CO2. Coronal slices (300 μm) containing the amygdala were made using a vibrating tissue slicer. The slices were incubated for 30 minutes at 35°C in artificial cerebrospinal fluid (ACSF) containing (in mM) 126.0 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.0 MgSO4, 2.0 CaCl2, 26.0 NaHCO3, and 10.0 glucose bubbled with 95% O2/5% CO2. After another 1 hour at room temperature, a single slice was transferred to the recording chamber.
Individual cells were visualized using a microscope (Axioskop2, Zeiss). Whole cell recordings were obtained in current clamp mode using a glass micropipette (1.5 mm OD, WPI) containing (in mM) 130 K-gluconate, 10 KCl, 10 HEPES, 2 MgCl2, 2 Mg-ATP, 0.2 Tris-GTP, 0.5 EGTA, and 5 phosphocreatinine. Temperature was maintained at 30°C. Signals were amplified using an AxoPatch 200B (Axon Instrument) and recorded onto the hard drive of an IBM-compatible computer. The calculated junction potentials (pClamp 10) were approximately 14 mV, but were not compensated in the reported membrane potential. All drugs were bath applied in ACSF with about 1 minute delay to reach the recording chamber. The cPSP events were detected with Minianalysis (Synaptosoft). To calculate the cPSP frequency ratio, the frequency at each minute was normalized to the baseline frequency (the mean cPSP frequency for the initial 5 or 10 minutes prior to experimental treatments). One-way repeated-measures ANOVA was used to test the main effect and the significance at each minutes compared with baseline value. Statistical significance was set at p<0.05 (Prism 4, GraphPad). All drugs were from Sigma (Sigma-Aldrich, St. Louis, MO).
RESULTS
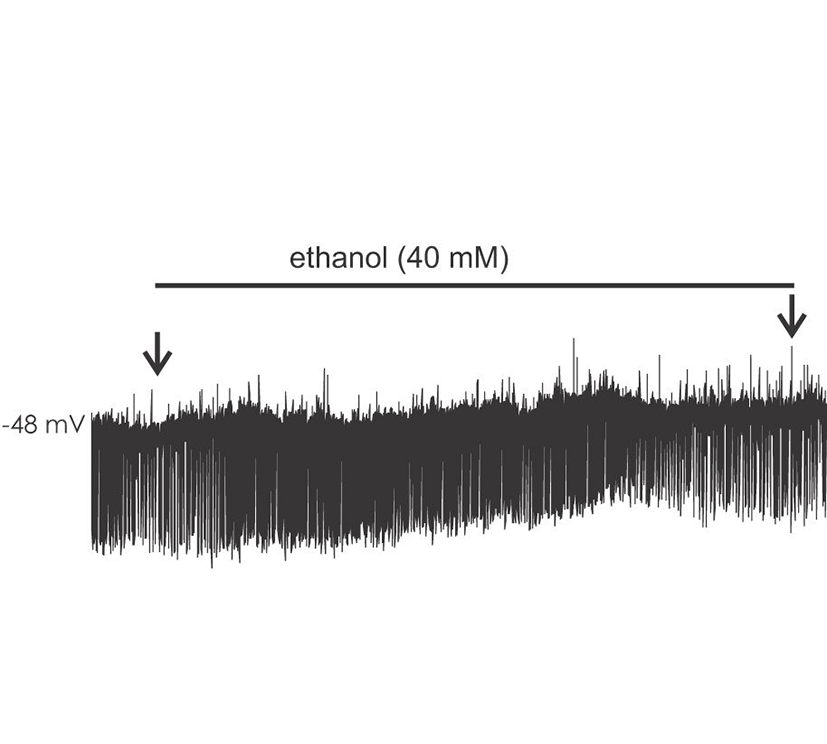
Ethanol modulated the circuit activity in BLA in a dose and time dependent manner. cPSPS in BLA was examined in slices from 16-23 days old rats (P16-23) (base cPSP frequency mean, 22.1±4.9; range, 8.8-49.6/minute, n=9). Acute application of 20 mM ethanol significantly increased the initial frequency ratio of cPSPs within 3 minutes (at 3 minutes after the application, 1.45±0.12). The frequency ratio of cPSPs subsequently decreased during washout (at 4 minutes after washout, 0.36±0.11). At 40 mM (base frequency mean, 29.3±4.2; range, 12.4-51.6/minute, n=10), the increase in frequency ratio cPSP was more pronounced (1.61±0.14), as was the decrease after washout (0.15±0.06). At 100 mM ethanol (base frequency mean 23.3±3.6; range, 10.2-33.6/minute, n=6), an initial frequency ratio increase was briefly present (2.12±0.35), but was followed by a strong attenuation (0.22±0.08) with a gradual return to baseline upon washout. Fig. 1.
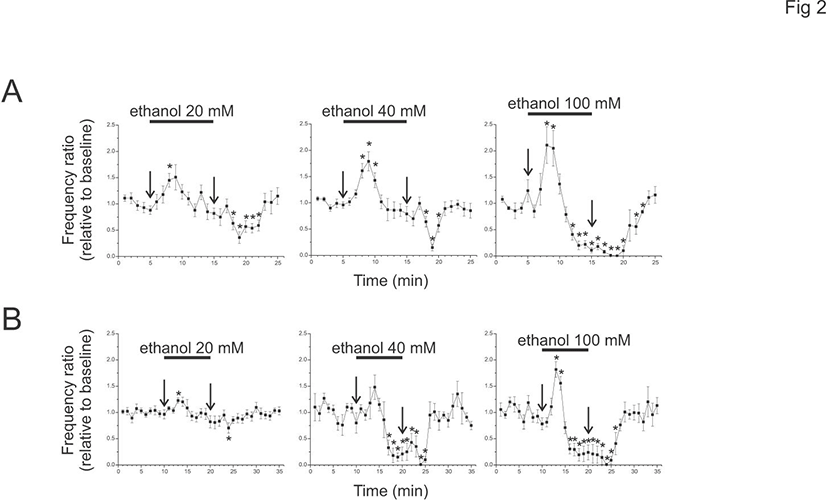
To test whether a similar ethanol effect is present in adult animals, ethanol of 20, 40, or 100 mM was applied to brain slices from P75-79 (Fig. 2). The cPSPs were present in adult animals (10 minute baseline, 20 mM, mean 36.7±4.1, range 14.2-55.3/minute, n=9; 40 mM, mean 33.2±6.4, range 19.6-54.2/minute, n=5; 100 mM, mean 23.8±2.2, range 16.6-28.4/minute, n=5). The peak enhancement of cPSP frequency ratio was larger than baseline at 20 and 100 mM (20 mM, 1.2±0.0; 100 mM, 1.82±0.15), but was not at 40 mM (1.48±0.23). However, during the ethanol application the frequency ratio was decreased at 40 and 100 mM (40 mM, 0.15±0.08 at 9 minutes after ethanol; 100 mM, 0.21±0.15), but not at 20 mM (0.82±0.12 at 10 minutes after ethanol). At 4 minutes after the start of washout, cPSPs were decreased at 20 mM and almost absent at 40 and 100 mM (20 mM, 0.70±0.01; 40 mM, 0.01±0.01; 100 mM, 0.01±0.01).
DISCUSSION
A recent work suggests that endogenous neuropeptides may modulate cPSP frequency in BLA, with anxiogenic peptides enhancing cPSP frequency and anxiolytic peptides reducing it (Chung & Moore, 2009). The current data indicate that ethanol has dose and duration effects on cPSP frequency that include both stimulation and inhibition. Biphasic effects of ethanol have been previously reported in humans and animals (Addicott et al., 2007; Pohorecky, 1977). In rats, ethanol injection both increased and decreased the release of noradrenaline and acetylcholine in temporal and dose-dependent manner (Rossetti et al., 1992; Stancampiano et al., 2004). The stimulating effect of ethanol may reflect the predisposition to alcohol addiction (Beckstead and Phillips 2009; Meyer et al., 2009). In this regard, it is important to note that ethanol stimulates dopaminergic neurons in ventral tegmental area (Okamoto et al., 2006; McDaid et al., 2008). Our data indicate that similar phenomenon may be present in BLA. In brain slices from young animals, low concentrations of ethanol (e.g. 20 mM) primarily increase BLA circuit activity, perhaps leading to positive behavioral effects such as increased arousal. Higher concentrations of ethanol (e.g.>40 mM) may inhibit BLA output which may be associated with anxiolysis and sedation.
Using a brain slice preparation, a related study reported that ethanol potentiates circuit activity in the form of giant depolarizing potentials (GDPs) which depend on GABAergic depolarization in hippocampal CA3 region (Galindo et al., 2005). This study used very young animal (3 to 7 days old) and the effect was only facilitatory. The ethanol effect was absent in animals more than 2 weeks old, specifically 18 to 23 days old. However this study is different from that study in both age and effect pattern. The age was either 2-3 or 10 week old animals, and although ethanol effects on cPSPs were apparent at both time points, the effects were distinct. While the effect of ethanol in slices from young animals was biphasic, effects in older animals were primarily inhibitory. Still, it is speculated that the cPSPs in amygdala and GDPs in immature hippocampus may share some basic mechanisms, such as GABAergic depolarization.
The finding that ethanol effects on cPSPs in slices from adult rats were mainly inhibitory is consistent with a general enhancement of GABAergic tone in mature rat brains, while neuronal circuit activity in immature amygdala may respond to ethanol with dose-and time- dependent facilitation, consistent with enhanced ethanol-induced arousal in young individuals (Silveri & Spear, 1998; Varlinskaya & Spear, 2002a, 2002b). These findings in amygdala roughly parallel electrophysiological data from hippocampal slices taken from immature and adult rats (Li et al., 2003). In general, ethanol enhanced spontaneous inhibitory neurotransmission to a greater extent in slices from adult animals compared to juvenile or adolescent animals. The sensitivity of GABAA receptor-mediated inhibitory processes to ethanol might increase with development from the juvenile period to adulthood. Such a process could explain the greater arousal and less sedation produced by ethanol in younger individuals (Silveri & Spear, 1998; Varlinskaya & Spear, 2002a, 2002b; Hefner and Holmes, 2007).