INTRODUCTION
Red spotted grouper, Epinephelus akaara, is a popular aquaculture species in tropical and sub-tropical areas. This species is known to be a protogynous hermaphrodite that first differentiates into female and changes to male later (Tanaka et al., 1990; Okumura, 2001; Li et al., 2006; Li et al., 2007; Kim et al., 2015). The success of seed production in this species is largely dependent on the timely supply of male because natural sex change takes place long time after the primary sex differentiation at around 5 to 6 years old (Li et al., 2006; Li et al., 2007). Thus, induction of artificial sex change at the time of primary sex differentiation could be a highly useful technique. To achieve this, however, further understanding on the process of primary sex differentiation is essential.
Classical concept of sexual differentiation in vertebrates suggests that the result of gonadal sex differentiation drives brain sex differentiation. It could be different particularly in the case of hermaphrodite fish. Serially sex changing teleosts demonstrate profound change in sexual behavior even before the gonadal trans-differentiation into a testis or an ovary indicating the influence of the brain (Grober & Sunobe, 1996; Kobayashi et al., 2013). There are huge amounts of evidences that support the involvement of the brain in the process of sex change in protogynous hermaphrodite (reviewed by Baroiller et al., 1999). However, the influence of brain in the process of primary sex differentiation in hermaphrodite fish has not been sufficiently investigated yet.
It is well known that the primary sex differentiation is under the influence of sex steroid hormones in fishes (Yamazaki, 1983; Hunter & Donaldson, 1983; Nakamura et al., 1998). Cytochrome P450 aromatase, a steroidogenic enzyme that converts androgens into estrogens, is also deeply associated with sex differentiation in fish (Kwon et al., 2001; Guiguen et al., 2009). This enzyme is encoded by two distinct genes (cyp19a1a: P450aromA-ovary type; cyp19a1b: P450aromB-brain type) in various fish species (Kwon et al., 2001; Kwon & Kim, 2013). The action of estrogen is mediated by estrogen receptor. However, the importance of estrogen receptor in the brain has not been properly explored so far with regard to gonadal sex differentiation in this species.
Several studies suggested that the importance of feedback regulation of gonadal steroids to orchestrate GnRH-GTH release, which subsequently regulate sex steroid production during gametogenesis, serial sex change and natural sex reversal (Peter et al., 1991; Grober & Sunobe, 1996; Goos et al., 1999; Zohar et al., 2010). As mentioned earlier, Baroiller et al. (1999) also suggested that the hypothalamic gonadotrophic axis may be needed to complete sex differentiation though not activate it.
The formation of the ovarian cavity is a characteristic landmark for the process of primary gonadal sex differrentiation in red spotted grouper (Kim et al., 2015). In many fish species, ovarian cavity are unequivocally discernible, allowing for the rapid identification of ovarian differentiation and detection any effects of estrogens or other sex steroids on gonadal differentiation (Dietrich & Krieger, 2009).
To find out the potential neuroendocrine influence on the primary sex differentiation of protogynous hermaphrodites, we investigated the expression of two aromatase genes (cyp19a1a and cyp19a1b), estrogen receptor alpha (esr1), GnRH and GnRH receptor genes (gnrh1 and gnrhr1) and three gonadotropin subunit genes (FSHβ fsh, LHβ lh and common glycoprotein alpha cga) in the brain of red spotted grouper during the formation of ovarian cavity.
MATERIALS AND METHODS
Red spotted grouper, E. akaara was obtained from Cheongsol aquaculture farm in Muan-gun, Jeonnam, Korea, and transported to a fish rearing facility in Sunmoon University. Fish were reared at 27±1℃ and 31± 1‰ in indoor tanks. Photoperiod was maintained at 14 hours light : 10 hours dark (14L:10D). Fish were fed a commercial diet (40 to 80 DPH, days post-hatch: Ottohime, Japan; 80 to 130 DPH: Myungsun, Korea) ad libitum twice a day. Sampling was carried out every 5 days between 40 to 105 DPH (n=7-8 at each sampling date). After that, fish were sampled additionally at 120 (n=4) and 130 DPH (n=3). These fish were anesthetized by 50 ppm benzocaine (Sigma, USA) and killed to remove whole brain including the pituitary. The brain of fish from 40 to 70 DPH was removed together with cranium and surrounding tissues because the size of the brain was tiny. The brain of fish from 75 to 130 DPH was removed only with the pituitary out of the head part.
Sequences for esr1, gnrh and gnrhr1 of red spotted grouper were not available in GenBank database since these genes have not been studied previously in this species. To identify these genes and obtain partial sequences, degenerate PCR was conducted using sequence information from the related species. Total RNA was extracted from the brain including the pituitary using TRIsure (Bioline, USA) and quantified using nanodrop-1000 (Thermo, USA). This RNA (1 μg) was reverse transcribed using TOPscritTM RT DryMIX (Enzynomics, Korea). The resultant cDNA was used as a template for subsequent degenerate PCR. Electrophoresis of PCR products were carried out in 1% agarose gel. All primers for degenerate PCR were designed using the Primer 3 software (version 2.2.3) and listed in Table 1. The degenerate PCR was carried out using GoTaqⓐ Green Master Mix (Promega, USA). The condition for degenerate PCR was as follows: initial denaturation at 95℃ for 5 min, 40 cycles of denaturation at 95℃ for 15 seconds, annealing at 60℃ for 15 seconds and elongation at 72℃ for 1 min. PCR products were then sequenced by 3730xl DNA Analyzer (Applied biosystems, USA).
Extraction, quantification and reverse transcription of total RNAs were the same as described earlier in this study. Expression of cyp19a1a, cyp19a1b, esr1, gnrh1, gnrhr1, fsh, lh and cga in the brain of red spotted grouper during the formation of ovarian cavity was investigated by quantitative real time -PCR (qRT-PCR) using the resultant cDNAs as templates. Primers for qRT-PCR were designed Designer software (Bio-Rad, Hercules, CA, USA), and listed in Table 2. The qRT-PCRs were carried out using Topreal™ qPCR 2× PreMIX SYBR Green (Enzynomics, Korea) and CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad). Abundance level of each mRNA was normalized against the amount of β-actin mRNA. Relative abundance was determined using the comparative threshold cycle method, 2−△△Ct, along with CFX Manager™ Software (Bio-Rad). Relative expression values of all samples were categorized and analyzed on the basis of day post-hatch and the status of ovarian cavity formation.
Fish were first fixed in 10% formalin for 12 hours. After removing the head and tail from the fixed fish, the remaining tissues (trunk part) were further fixed in 10% formalin again for another 12 hours. The fixed tissues were then dehydrated with ascending alcoholic series, cleared in xylene, and embedded in paraffin wax. Tissues were cut into a thickness of 5-8 μm, stained with haematoxylin & eosin. Tissue sections were observed under light microscope (DM500, Leica, Germany) to judge the status of ovarian cavity formation individually.
Data were presented as mean±SEM. Statistical differences of mRNA expression between different DPH was analyzed by Mann-Whitney U-test (P<0.05). Statistical differences of mRNA expression levels between different phases of ovarian cavity formation was analyzed by one-way ANOVA and Games-Howell range tests (P<0.05). Statistical analyses were performed using SPSS version 18.0.
RESULTS & DISCUSSION
Degenerate PCR successfully amplified a prominent band for each gene from the brain of red spotted grouper. The size of PCR product for esr1, gnrh1 and gnrhr1 were 523, 252 and 884 base pairs (bp), respectively (Table 3). The sequences of these fragments exhibited 96-99% sequence identity to estrogen receptor alpha (accession number: HQ662335, HM030760) and GnRH-receptor1 (accession number: DQ536435) of other grouper species such as E. coioides, E. adscensionis, E. fasciatus.
Based on the gonadal histology, the major period of ovarian cavity formation was found to be 65 to 105 DPH in this species. All genes investigated in this study were detectable in the brain earlier than this period (40 to 60 DPH) but not high enough to compare with the expression levels during the ovarian cavity formation.
P450aromA mRNA (cyp19a1a) in the brain significantly increased at 70 and 90 DPH from the respective previous sampling date (Fig. 1). The levels tended to increase as the formation of ovarian cavity proceed but this increase was not statistically significant (P>0.05). P450aromB mRNA (cyp19a1b) increased significantly from 75 DPH and remained high during the rest of period investigated. As the formation of ovarian cavity proceed, the level of cyp19a1b expression increased significantly (Fig. 2, P< 0.05). Our findings are in agreement with previous studies for other fish species where cyp19a1a was contributable to gonadal sex differentiation (Kwon et al., 2001; Patil & Gunasekera, 2008; Guiguen et al., 2009) wherase cyp19a1b was highly expressed in the brain and might cause the brain sex differentiation (Vizziano-Cantonnet et al., 2011). However, highly expressed cyp19a1b in the brain is not likely to be the cause of the ovarian cavity formation since it was low at the onset of the formation.
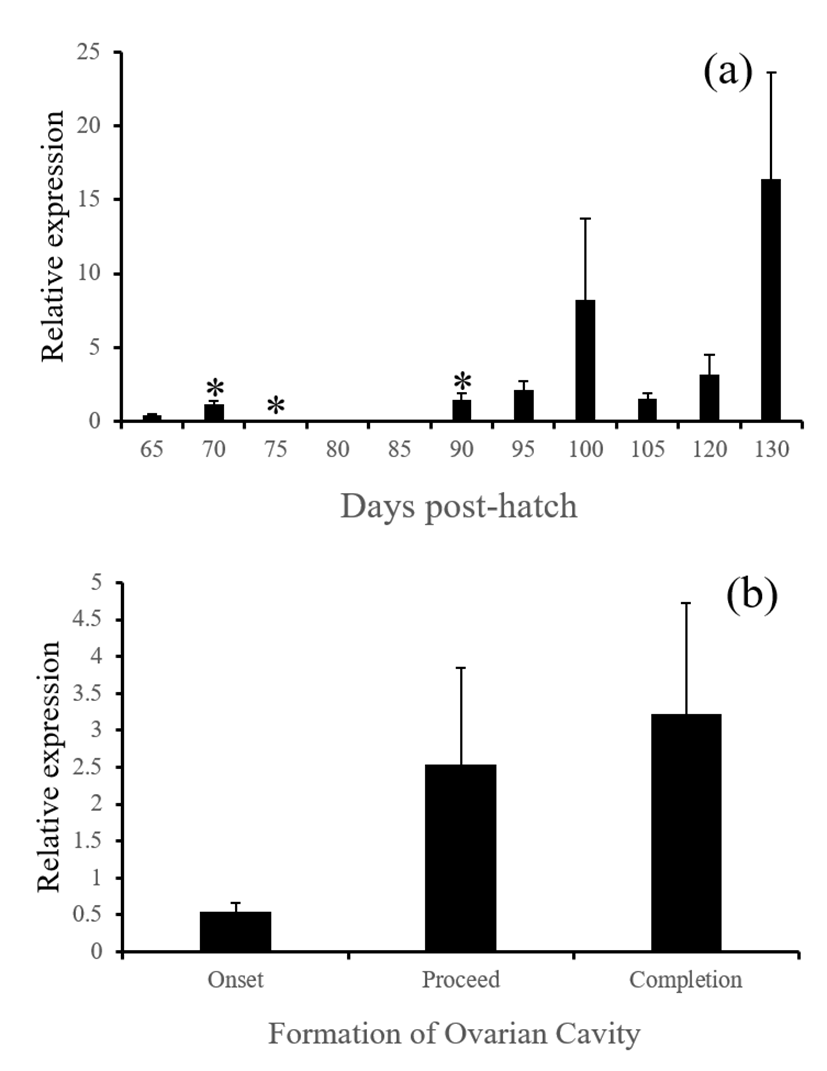
Estrogen receptor alpha mRNA (esr1) in the brain of red spotted grouper significantly increased from 90 DPH and remained high during the rest of period investigated. As the formation of ovarian cavity proceed, the level of esr1 expression increased significantly (Fig. 3, P<0.05). Brain aromatase activity is correlated to sex steroid levels, and the high expression of cyp19a1b is associated to an autoregulatory loop through which estrogens and aromatizable androgens up-regulate aromatase expression. This process requires estrogen receptor binding on an estrogen response element located on the cyp19a1b promoter (Diotel et al., 2010). These together suggest active production of estrogens and auto-regulation in the brain at the time of ovarian cavity formation. In supporting of this, Nakamura & Nagahama (1985) have noticed the presence of steroid producing cells at the beginning of ovarian cavity formation in a fish species.
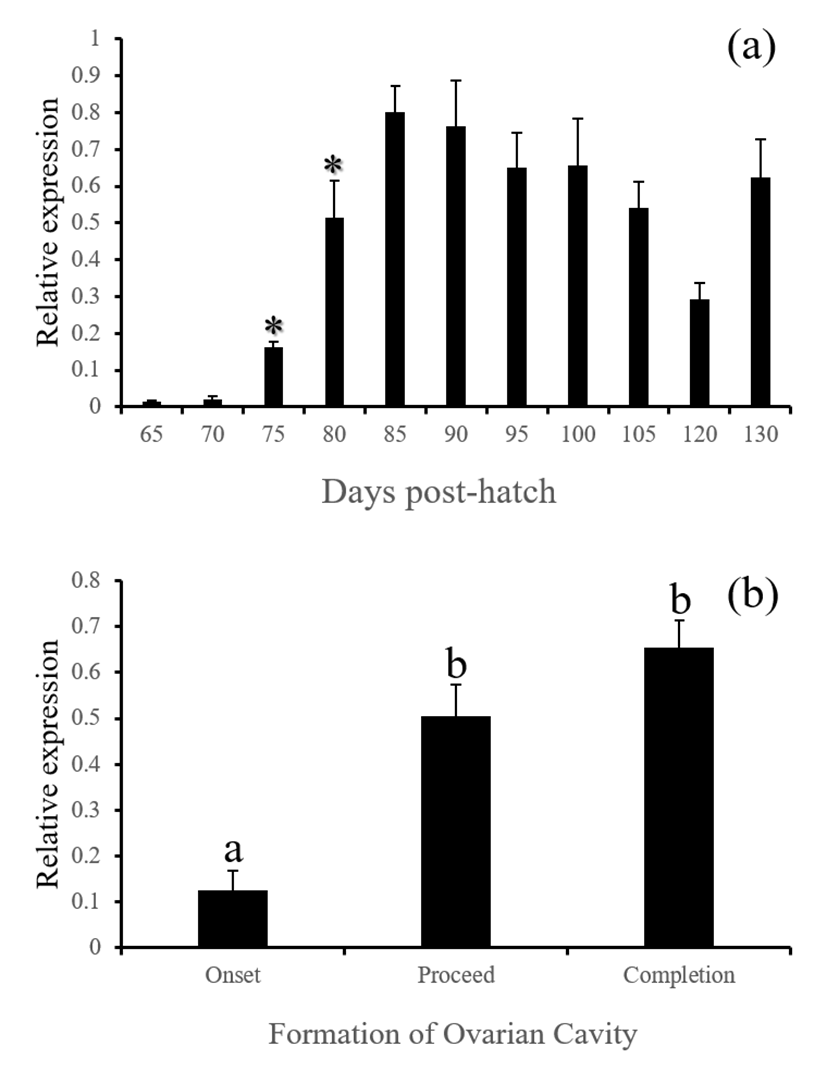
gnrh - GnRH mRNA in the brain significantly increased at 70 DPH and remain suppressed until 90 DPH (Fig. 4, P<0.05). Since then, it recovered back to the level before 70 DPH. However, the levels did not show any differences with regard to the formation of ovarian cavity (P>0.05). In fish including red spotted species in this study, the strong expression of gnrh appear to be in favor of testicular differentiation rather than ovarian differentiation. Nile tilapia showed clear differential temporal patterns of GnRH localization and expression between genetic male and genetic female larva (Swapna et al., 2008). In European sea bass, the expression of GnRH gene was signicantly higher in male-dominant population than female-dominant population (Moles et al., 2007). Suppressed expression of gnrh in this study are consistent with these findings, signifying potential hypothalamic influence on the primary sex differentiation in this species.
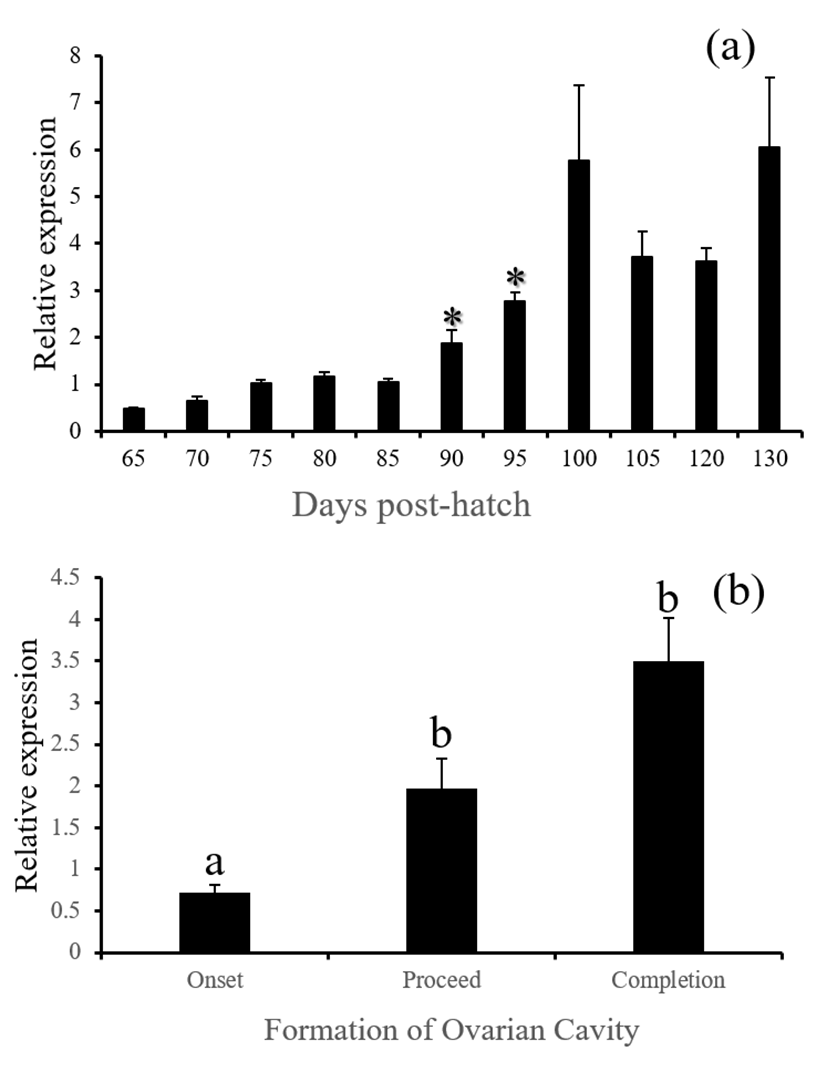
gnrhr1 - GnRH-receptor 1 mRNA in the brain was started to be actively expressed from 90 DPH and remained high since then. This increased level seems associated with the formation of ovarian cavity as the level was significantly higher at the phase of completion than the level at the onset (Fig. 5, P<0.05).
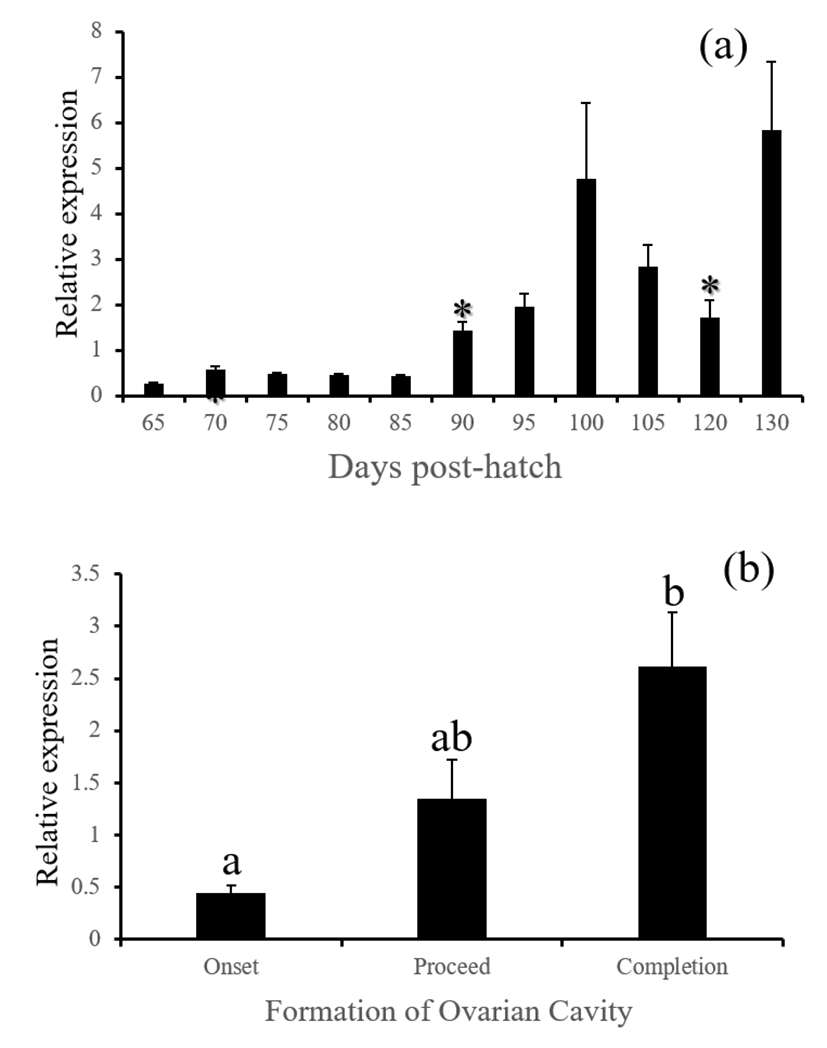
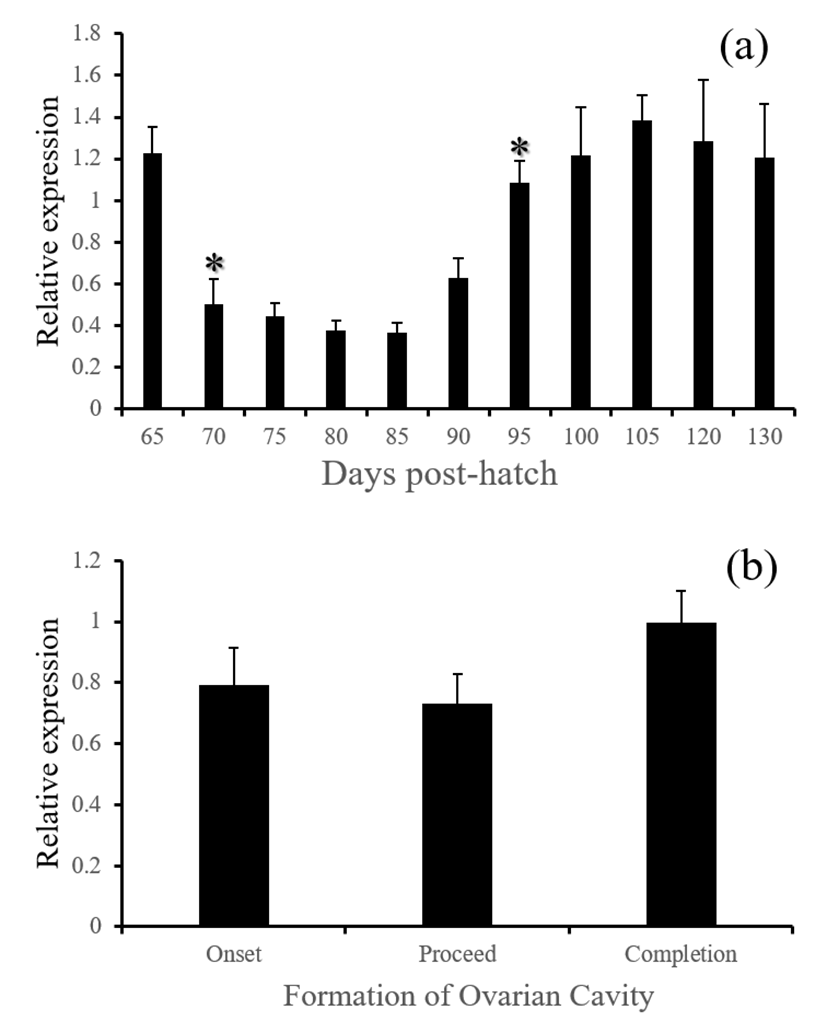
fsh, lh and cga - These three GtH subunit genes in the brain were started to be actively expressed from 85 (for lh and cga) or 90 DPH (for fsh). Expression of these genes were associated with the formation of ovarian cavity, showing significantly higher levels at the phase of completion (Fig. 6, 7 and 8, P<0.05).
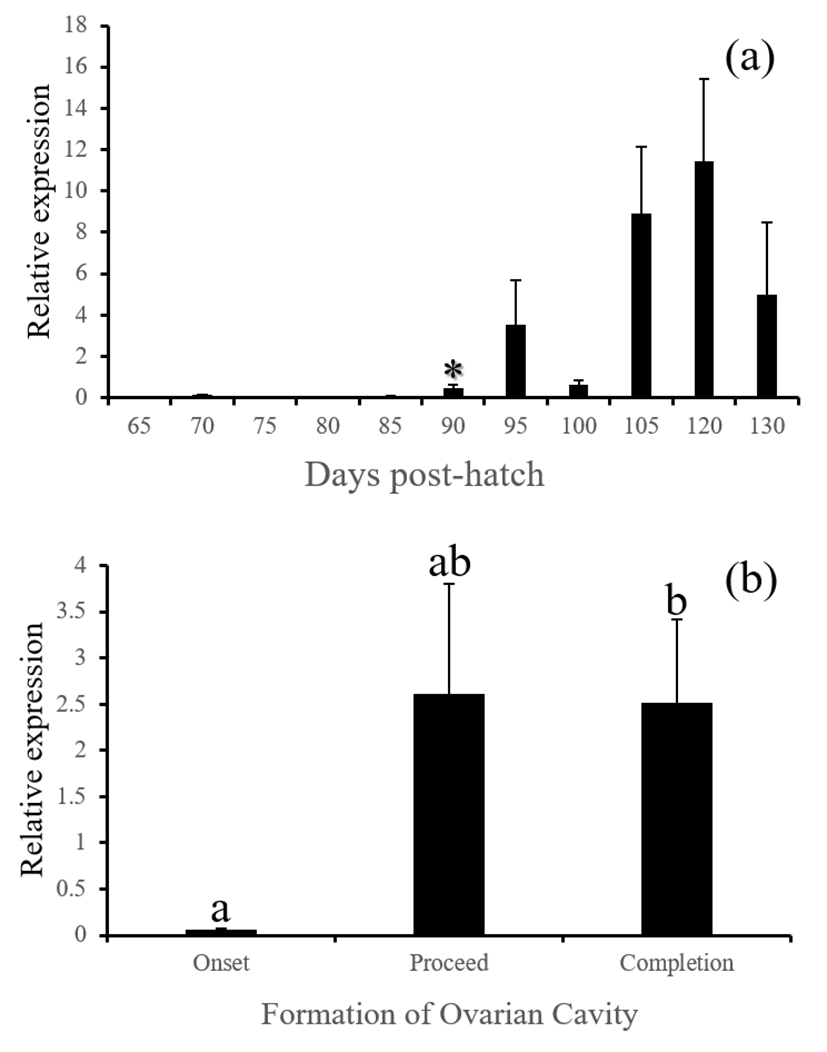
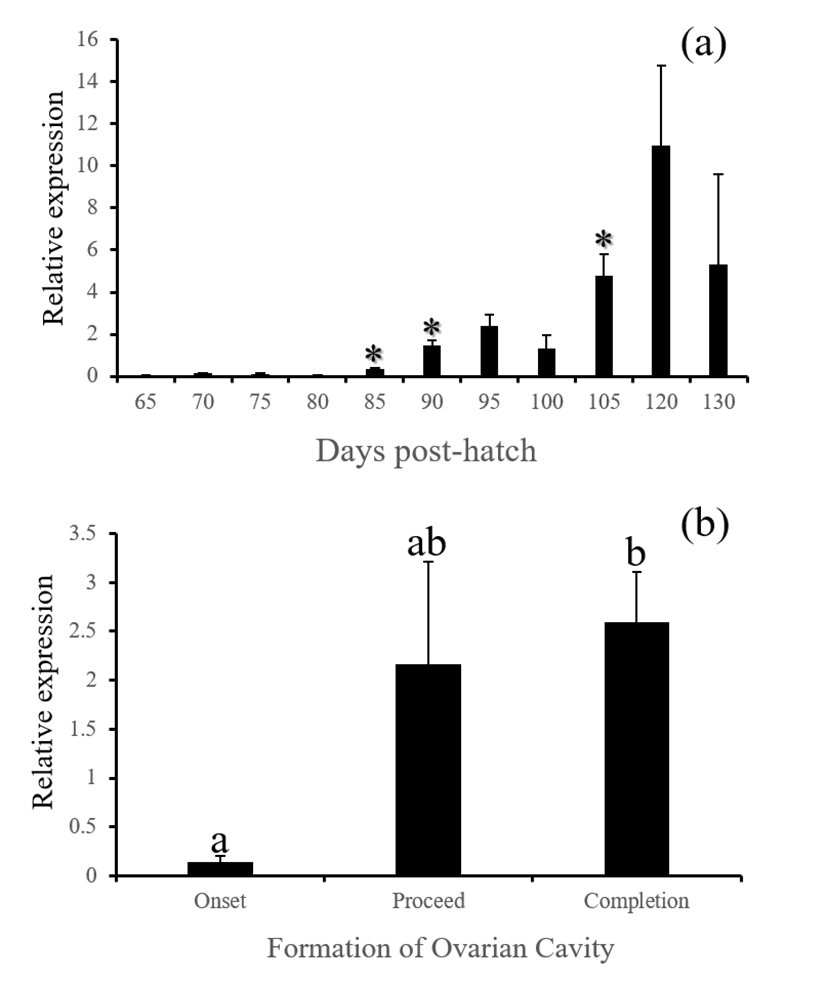
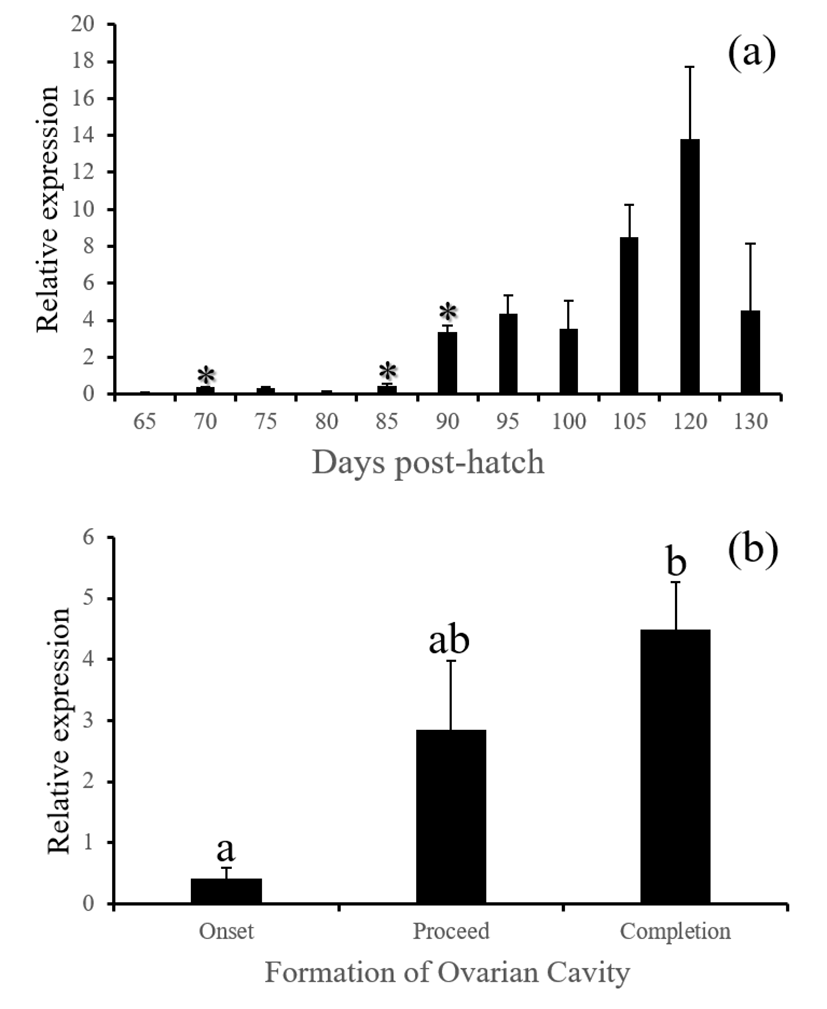
GnRH receptor in the pituitary relays hypothalamic signals to gonadotropic cells to induce the production of GtH. Similar expression patterns of gnrhr1, fsh, lh and cga observed in this study is well agreed to this flow of hormonal signals in animals. Expressions of all these genes were closely associated with the formation of ovarian cavity although none of these were initiating factors for the sex differentiation. Baroiller et al. (1999) suggested that the hypothalamic gonadotrophic axis may be needed to complete sex differentiation but not activate it. The hypothalamic gonadotropic influence on fish sex is particularly obvious in hermaphrodite fish. In a protogynous species the bluehead wrasse, Thalassoma bifasciatum, injection of females with human chorionic gonadotropin (hCG) induced in all cases a significant percentage of female-to-male sex inversions, demonstrating that a short rise in gonadotropic secretion will trigger sex inversion without the need for high sustained secretions (Koulish and Kramer, 1989). Red spotted grouper is a protogynous like the bluehead wrasse, and showed siginificant decrease of gnrh and increase of gonadotropic factors such as gnrhr1, fsh, lh and cga during the formation of ovarian cavity, suggesting that the primary sex differentiation of this species might be sensitive to artificial treatment with GnRH or GtH.
In conclusion, highly expressed cyp19a1b in the brain is not likely to be the cause of the ovarian cavity formation since it was low at the onset of the formation. Active production of estrogens and auto-regulation may take place in the brain at the time of ovarian cavity formation in this species. The hypothalamic gonadotropic influence on fish sex seems obvious in hermaphrodite fish. Red spotted grouper is a protogynous, and showed siginificant decrease of gnrh and increase of gonadotropic factors such as gnrhr1, fsh, lh and cga during the formation of ovarian cavity, suggesting that the primary sex differentiation of this species might be sensitive to artificial treatment with GnRH or GtH. Further studies should prove this by means of in vivo experiment.
