INTRODUCTION
Castration can be scientifically defined as extirpation or suppression of testicular function, and can be classified as surgical or chemical (Neto et al., 2014). Setting aside human applications, surgical castration (also known as orchidectomy, ORX) has been frequently performed to avoid uncontrolled breeding of domestic and/or companion animals and to elucidate the androgen-related issues in biomedical researches. Although the ORX method guarantees the perfect sterilization and sufficient testosterone (T) deprivation, it also has several disadvantages such as requirement of anesthesia, unwanted bleeding and infections (Jana & Samanta, 2006). Therefore substantial number of studies has been conducted to develop chemical castration method for a better alternative to the surgical procedure.
In general, chemical castration uses pharmaceutical drugs such as anti-androgens, steroidogenesis inhibitors and GnRH analogs (Attar et al., 2009). These drugs were chosen based on the strong backgrounds in endocrinology (i.e., hypothalamus-pituitary-testis hormonal axis), but they also have some drawbacks such as high cost, side effects (i.e., bone loss) and poor long-term efficacy (Costantino et al., 2014). Vaccines against GnRH have been reported to suppress reproduction in dogs (Jung et al., 2005), but these approaches are unable to provide permanent sterility (Rhodes, 2017).
Over the last few decades, several laboratories have developed more reliable chemical castration methods, using bilateral intratesticular injection (BITI) of simple chemical solutions such as glycerol (Wiebe & Barr, 1984), lactic acid (Fordyce et al., 1989), calcium chloride (CaCl2; Jana & Samanta, 2007; Jana et al., 2002), zinc gluconate (Oliveira et al., 2012), and hypertonic saline (20% NaCl; Emir et al., 2008; Emir et al., 2011; Kwak & Lee, 2013). However, the main goal of the studies was an effective sterilization of non-experimental animals for birth control. Therefore the 'androgen-depleting' potential of the BITI method which may replace ORX procedure in the basic researches has not been examined at all. Indeed, rodent model of androgen deprivation by ORX has been exclusively used in numerous biomedical studies to understand the exact nature of androgen actions on the development, differentiation and physiology of target tissues (Smith et al.,2005; Durán-Pastén et al., 2013).
We hypothesized that the chemical castration by hypertonic saline BITI might have same or equivalent efficacy to surgical castration, so both methods might induce similar responses in the androgen-sensitive tissues. In the previous study, we demonstrated that the hypertonic saline BITI caused the very similar biochemical changes (i.e. genomic DNA degradation patterns and gel electrophoretic protein patterns) in the accessory sex organs as shown in the ORX rats (Kwak & Lee, 2013). Present study was undertaken, for further evaluation at transcriptional level, to compare the effects of both ORX and hypertonic saline BITI on changes in hormone gene expressions of hypothalamus and pituitary.
MATERIALS & METHODS
Male Sprague-Dawley rats (5 months old) were obtained from Han-Lim Animal (Gyunggi-do, Korea) and acclimated 1 week in our animal facility under conditions of 12-h light/dark cycle (lights on at 07:00 h) and constant temperature of 22±1℃. Animal care and experimental procedures were approved by the Institutional Animal care and the use committee at the Sangmyung University (R-1301) in accordance with guidelines established by the Korea Food and Drug Administration.
Rats were randomly divided into three groups including intact group (Intact), ORX group (ORX) and hypertonic saline BITI group (SAL). Sterilized 20% saline was directly injected into the animals (750 μL per testis) using 1 mL syringes. Bilateral ORX was performed at the same day of the saline injection. All rats were sacrificed at 4 weeks after the ORX or saline injection. The serum was collected for T ELISA, and the tissues were used for total RNA extraction.
Serum T levels were measured by a competitive ELISA kit according to manufacturer’s instructions (ab108666; Abcam, USA). The assay sensitivity was 0.07 ng/mL, and the detection range was 0.2-16 ng/mL.
Total RNAs were isolated from tissue samples using the single-step, acid guanidinum thiocyanate-phenol-chloroform extraction method. Total RNAs were used in RT-PCR reactions carried out with Maxime™ RT PreMix (InTron, Korea) and Accupower PCR Premix (GeneAll, Korea) according to the manufacturer’s instructions. Sequences of the primers and the specific PCR conditions used in this study are listed in Tables 1 and 2, respectively. The reaction products were analyzed by gel electrophoresis in 1.5% agarose gel (75V, 65min) and visualized by ethidium bromide staining. The band intensities were measured using the image analysis system (ImagerⅢ-1D main software, Bioneer, Korea). Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) PCR was used as reference standard for normalization of quantitative RT-PCRs in the present study.
RESULTS
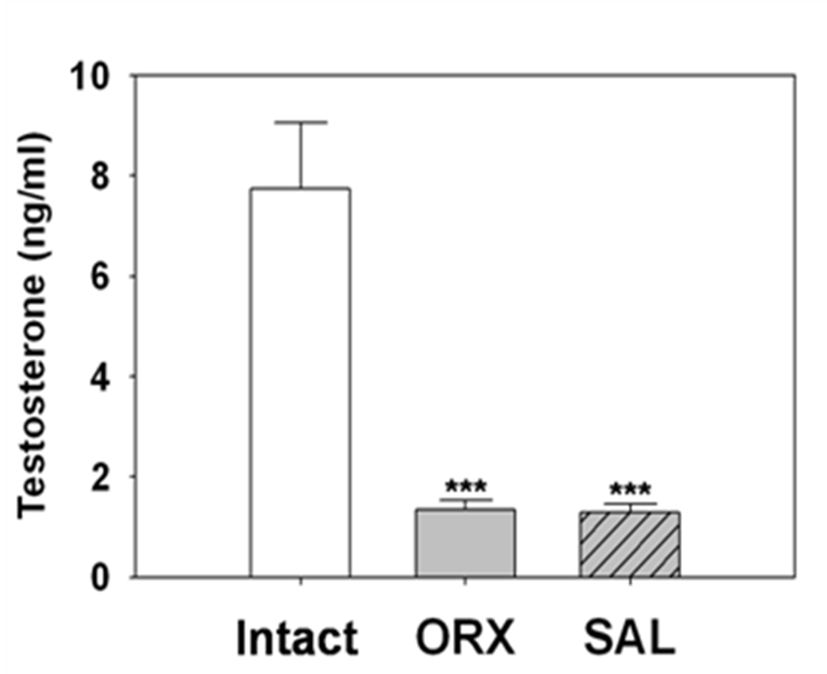
Fig. 1 shown that the serum T levels of ORX rats after 4 weeks of surgery exhibited a significantly drop (1.34±0.19 ng/ml, p<0.001) as compared with the levels of intact animals (7.74±1.31 ng/mL). Similarly serum T levels of hypertonic saline BITI animals (SAL) shown significant decrease (1.28±0.18 ng/mL, p<0.001) as compared with intact animal levels. There was no statistical difference between the T levels of ORX group and SAL group animals.
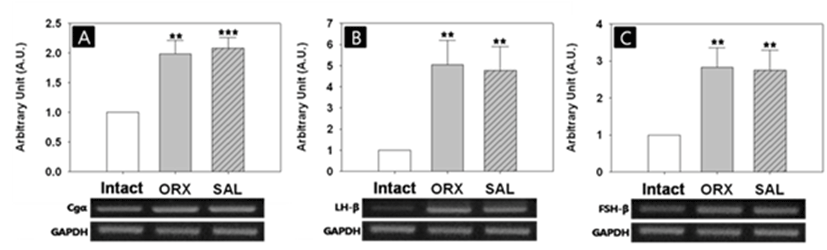
To elucidate whether the hypertonic saline BITI method possesses equivalent potential with ORX method on the target gene expression at transcription level, we performed
RT-PCRs using the pituitary total RNA samples. In general, both ORX and BITI method induced similar stimulatory effects on the pituitary gonadotropin gene expressions (Fig. 2A, B, C). Four weeks after castration, the mRNA level of gonadotropin subunit alpha (Cgα) was significantly increased (1:1.99±0.22 AU, p<0.01). Likewise, the Cgα mRNA level of hypertonic saline BITI group was significantly increased (1:2.08±0.18 AU, p<0.001). We found no difference between the levels of ORX and BITI groups. The levels of LH-β mRNAs were also significantly increased in both ORX group and hypertonic saline BITII group (1ntact: ORX:SAL = 1:5.04±1.15:4.76±1.14 AU, p<0.01). The levels of FSH-β transcript also shown similar stimulatory results in ORX group and hypertonic saline BITI group (1ntact:ORX:SAL = 1:2.84±0.53:2.76±0.54 AU, p<0.01).
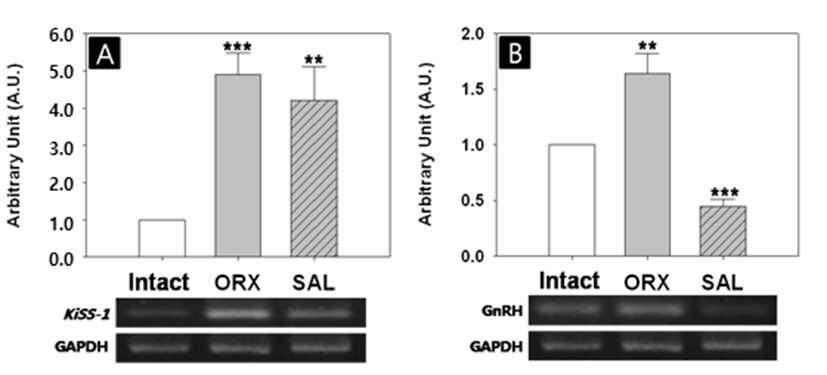
The effects of two castration methods on hypothalamic gene expressions varied depending on the genes (Fig. 3). The Kiss-1 mRNA level was significantly increased in ORX group (1:4.91±0,58 AU, p<0.001, Fig. 3A). Similarly, the Kiss-1 mRNA level was significantly increased in hypertonic saline bITI group (1:4.20±0.91 AU, p<0.05). There was no difference between the levels of ORX and BITI groups. The GnRH mRNA level of ORX group was significantly higher than that of intact group (1:1.64±0.19 AU, p<0.01, Fig. 3B). Interestingly, the GnRH mRNA level of BITI group was significantly lower than that of intact group (1:0.45±0.06 AU, p<0.001), showing inverse relationship between the two groups.
DISCUSSION
The present study aimed to investigate whether the chemical castration with simple salt solution can be a substitute for surgical castration in the preparation of androgendeprivation animal model. In particular, we compared the effects of the two castration methods on the hormone expressions of pituitary and hypothalamus at transcriptional level. To confirm whether the androgen deprivation occurred sufficiently, we measured the serum T levels of the two groups. The serum T levels were dramatically decreased in both ORX group and hypertonic saline injection group after 4 weeks of the manipulations. The suppression rates, less than 1/6 of intact level, were similar in the two groups. This finding was in good agreement with previous studies, showing complete ablation of Leydig cell populations in the testis of hypertonic saline- or calcium chloride-treated rats (Jana et al., 2002; Emir et al., 2008).
Concerning the injection materials used in simple chemical castration methods, the use of calcium chloride anteceded that of sodium chloride. In rats, serum T level was dramatically reduced after 4 weeks post-injection in a dose- dependent manner (5-20 mg/testis/100g body wt in 0.1 mL saline) by single calcium chloride BITI (Jana et al., 2002). The authors also demonstrated similar dose-dependent (5-20 mg/testis/kg body wt in 1mL saline) decrease of serum T level in dogs after 45 days post-injection (Jana & Samanta, 2007). In contrast, chemical castration with 20% calcium chloride injection into donkey testes failed to induce marked decrease in serum T; only 45% of initial serum level after 1 month post-injection, and the histopathological study confirmed the presence of proliferative Leydig cells (Ibrahim et al., 2016). These results suggest that the castration effect of calcium chloride injection may vary depending on the species. Previous study and our present result demonstrate both calcium chloride and sodium chloride could have similar capability of androgen deprivation in rats, when the same hypertonic condition (20%) was applied (Jana et al., 2002). It is intriguing that BITI of 20% calcium chloride in 95% ethanol as diluent instead of saline or lidocaine revealed improved sterilization and T deprivation in dogs (Leoci et al., 2014).
It is well known that gonadal steroids exert direct negative feedback effects at the pituitary as well as at the hypothalamic level in rodents (Kalra & Kalra, 1983). In male rats, ORX can cause an increase in serum LH concentrations, and T replacement can suppress these levels (Kalra, 1985). ORX also induces the changes in transcriptional levels of pituitary and hypothalamic hormones. In the present study, both ORX and hypertonic saline injection significantly increased mRNA levels of pituitary Cgα, LH-β and FSH-β by similar extents. Likewise, hypothalamic KiSS-1 mRNA levels were significantly increased by the two castration procedures. These findings are in line with the ORX effects on expressions of gonadotropin subunits and KiSS-1 in previous studies (Dalkin et al., 2001; Castellano et al., 2009), and indicates the two procedures could have equivalent potential in the regulation of the hormone gene expressions. However, changes in hypothalamic GnRH mRNA levels in response to the two castration procedures are opposite in our study; significant increase in ORX group and significant decrease in hypertonic saline injection group. Indeed, effects of ORX on the hypothalamic GnRH expression in male rats have been disputable issue. As a consequence of ORX, GnRH mRNA levels are either unchanged (Wiemann et al., 1990) or decreased (Rothfeld et al., 1987) in male rats. Making things more complicated, recent study reported that ORX elevates hypothalamic GnRH expression (Spratt & Herbison, 1997), and this result coincides with our finding. On the other hand, our hypertonic saline injection study disclosed significant decline in GnRH mRNA. We speculate about plausible causes for this huge discrepancy in hypothalamic GnRH expression between ORX group and hypertonic saline injection group. First, the levels of hypothalamic GnRH transcript seem to be fluctuated after androgen deprivation (Emanuele et al., 1996), and the expression profiles could be temporally different between these two groups. Second, unlike in ORX animals, relatively high level of phagocytosis will be maintained for some period in the testes of chemically castrated animals. This pathophysiological state will carry over the systemic elevation in inflammatory cytokine and corticosterone which can play as modulators of hypothalamic GnRH expression (Wu & Wolfe, 2012; Gore et al., 2006). Third, some reports insist the presence of 'direct neural pathway between the hypothalamus and the testes' that modulates T secretion independently of the pituitary (Selvage & Rivier, 2003; Selvage et al., 2006; James et al., 2008). The degree of this testicular denervation could be different between the two castration procedures, expecting 'instant full denervation' in ORX animals while 'delayed full denervation' in hypertonic saline injection group. This temporal gap may cause the differential expressions of hypothalamic genes in the two groups. Further studies, allowing these aspects, are necessary in order to evaluate the hypertonic saline BITI method as a substitute for ORX in preparation of animal model for investigating the androgen effects on the hypothalamic target gene expressions.
Growing number of evidence shows that chemical castrations by BITI of simple salt solution have arrived at a reliable sterilization method for rat, dog and cattle (Jana & Samanta, 2006; Jana & Samanta, 2007; Fagundes et al., 2014; Neto et al., 2014). Astonishingly, no scientific trial has been undertaken into the androgen deprivation potential of this simple chemical castration method as a substitute for ORX in biomedical researches. Actually, numerous studies have employed 'ORX and/or T replacement animal model' to clarify the physiological roles of androgen (Valenti et al., 1997; Lightfoot, 2008; Inada et al., 2011). Nonetheless, chemical castrations by BITI of simple salt solution could have certain advantages over surgical castration. Surgical castration is a time- and cost-consuming procedure, should be performed by experts, and it also imposes burdens on animals with unhealthy conditions. During and after the surgery, the animals could be improperly damaged by anesthesia and unexpected infection. Furthermore, the surgical procedure itself tends to be an animal welfare violation. Our study revealed there were no detectable changes in the general health status of the hypertonic saline-injected animals with the exception of testicular atrophy, and daily consumption of food and water remained unaffected. So minimum safety of hypertonic saline BITI method could be guaranteed.
We previously demonstrated that hypertonic saline BITI can induce ORX-like T deprivation effects on accessory sex organs such as weight loss of the epididymis, and identical protein patterns on SDS-PAGE (Kwak & Lee, 2013). Taken together, despite the lack of available strong evidence-based data on the mechanisms of the testicular cell death and T deprivation, the present study suggests the chemical castration by hypertonic saline BITI could be a promising substitute to conventional surgical castration. Effort to improve the safety and efficiency as well as extension of target tissue coverage of the chemical castration with simple salt solution will be worth in near future, particularly in biomedical researches.
