INTRODUCTION
Aquaculture of high-value marine finfish species continues to develop rapidly in Korea. Grouper (members of the Family Serranidae, Subfamily epinephelinae) is one of the most commercially high-priced marine fish in the live fish markets (McGilvray & Chan, 2002). Particularly, the red spotted grouper, Epinephelus akaara (Temminck & Schlegel), has been recognized as economically important species in Korean Peninsula, Japan, China and Southeast Asian countries (Cho et al., 2015). Although many attempts have been made to produce red spotted grouper larvae and juvenile due to high commercial value and consumer demand, high mortality in early life stages and high incidence of skeletal deformities are critical problems (Sugama & Ikenoue 1999; Song et al., 2005).
Marine fish larvae undergo major functional and morphological changes during successive developmental stages. Most malformations develop during early developmental stages. Therefore, skeletal deformities of marine fish larvae can affect successive development, decreasing their quality and commercial value (Cahu et al., 2003). Lall & Lewis-McCrea (2007) suggested that deficiencies of some vitamins and certain minerals can be correlated with severe skeletal deformities in fish larvae under culture conditions. Particularly calcium and phosphorus in bone and blood have been used as indicators of development and maintenance of skeletal system. Calcium deficiency causes a delay in ontogeny of skeletal development and insufficient phosphorus leads to skeletal abnormalities (Fontagne et al., 2009). In addition, estrogen concentrations are closely linked to skeletal growth of fish (Sasayama, 1999; Persson et al., 1999; Suzuki et al., 2000).
Skeletal deformities were already described in other groupers, like seven-band grouper (E. septemfasciatus, Nagano et al., 2007), red (E. akaara, Setiadi et al., 2006; Park et al., 2016) or orange (E. coioides, Cruz-Lacierda et al., 2000) spotted groupers. Most studies in skeletal development of E. akaara have mainly focused on skeletal development; development of external morphology (Mito et al., 1967), development of fins and scales in larval and juvenile stages (Fukuhara & Fushimi, 1988), development of jaws and feeding (Kayano, 1988). However, little information has been reported on the skeletal deformity and bone mineralization, and their interactions with E. akaara. Therefore, objective of this study is to investigate the relationship between skeletal deformities and plasma calcium, phosphorus, and estradiol-17β levels in red spotted grouper juveniles.
MATERIALS AND METHODS
Red spotted grouper (at 110, 140, 180, 300 DAH) in four different groups were obtained from Marine Science Institute of Jeju National University. Mean total length and weight 6.97±0.15 cm and 5.87±0.10 g, 7.13±0.80 cm and 7.09±2.52 g, 8.29±0.66 cm and 8.75±1.80 g, 11.65±1.45 cm and 26.53±8.34 g (Table 1). The fish were reared in glass aquaria with running seawater of 34±0.5 ‰ at water temperature of 15.0±1.0℃. The fish were fed artificial feed twice a day, but were not feed 24 h prior to the experiment. During the experimental period, the salinity, dissolved oxygen, and pH were maintained at 34±0.5 ‰, 6.5±1.0 mg/L, and 7.8±0.3, respectively. We classified red spotted grouper juveniles depending on the occurrence of deformity. For physiological analysis, blood was taken immediately from caudal vein with a heparinized 1 mL syringe. A total of 83-170 samples were taken in each groups.
| Group | Days | Total length | Total weight |
|---|---|---|---|
| (DAH) | (cm) | (g) | |
| Group 1 | 110 | 6.97±0.15 | 5.87±0.10 |
| Group 2 | 140 | 7.13±0.80 | 7.09±2.52 |
| Group 3Group 4 | 180300 | 8.29±0.6611.65±1.45 | 8.75±1.8026.53±8.34 |
For osteological observation on external shape, the specimens were photographed from left lateral view using a digital camera (NIKON D300s, Nikon Co., Japan) with a resolution of 12.3 megapixels. Fish were classified by the criteria of the appearance of macroscopically visible body deformations. In addition, the specimens were used for observation of skeletal structures by soft X-ray photographs (SFX-130, SOFTEX Co., Japan).
Collected blood was centrifuged for 15 min at 13,000 rpm at 4℃. Total plasma calcium and phosphorus in normal and malformed fish were measured respectively using biochemistry automatic analyzer (FUJI DRI-CHEM NX 500i, Fujifilm Co., Japan). Plasma estradiol-17β (E2) level was measured by radioimmunoassay (RIA) according to the Kobayashi’s method (Kobayashi & Mikuni, 1987). Antiserum for E2 was purchased from Cosmo-Bio Co., Ltd. (Tokyo, Japan). Radio-labeled steroids ([2,4,6,7-3H]-E2) was purchased from Amersham Lifesciences (England). For the assay, steroids from plasma were extracted twice in 2 mL diethyl ether, dried under nitrogen gas and were dissolved in 0.1% gel-PBS.
RESULTS
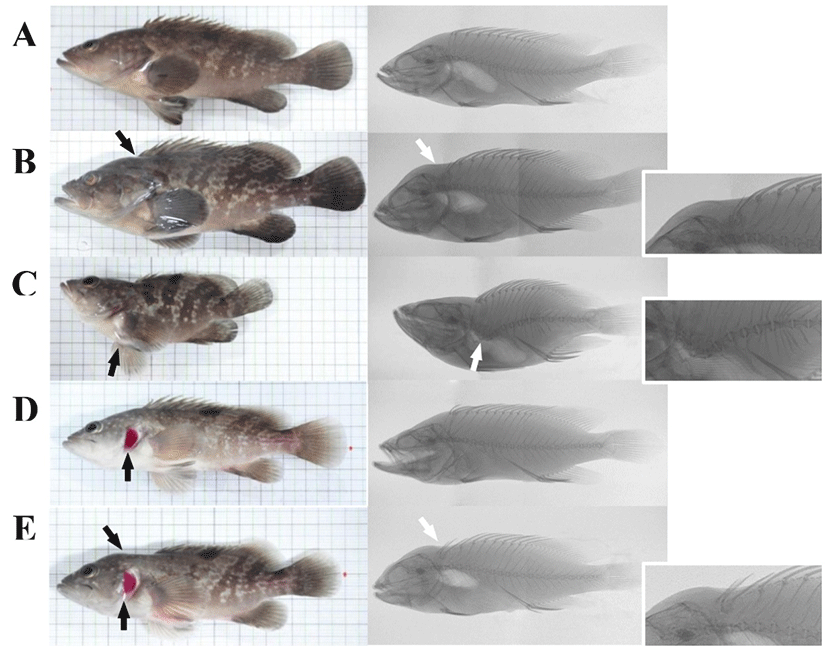
There were different deformity types in E.akaara. Morphometric analysis of skeleton in red spotted grouper are presented in Fig. 1. The cephalic (Fig. 1-B), vertebral (Fig. 1-C), opercular (Fig. 1-D) deformity and complication (Fig. 1-E) occurred most frequently in deformed E. akaara (Fig. 1). The incident rate of normal and deformed fish was shown in Table 2. Results of normal were 80.5% of group 1 (110 days), 78.1% of group 2 (140 days), 76.1% of group 3 (180 days), and 75.0% of group 4 (300 days). The incidence depending on deformity patterns showed 6.1%, 4.3%, 4.3%, and 3.0% of cephalus, 4.9%, 2.4%, 4.3% and 4.0% of deformity in vertebrae, 4.9%, 8.5%, 5.1% and 8.0% of operculum, 3.6%, 1.8%, 8.5% and 5.0% of complication deformational symptoms in group 1, group 2, group 3, and group 4 respectively in red spotted grouper, E. akaara.
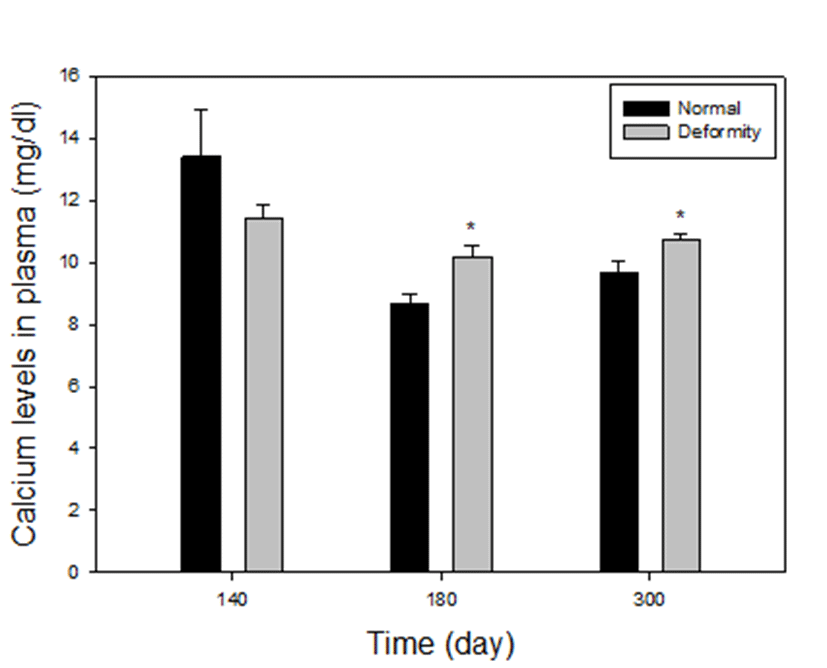
Plasma calcium levels between normal and deformed fish at 140, 180 and 300 days after hatching, are presented in Fig. 2. In comparison between normal and deformed fish, it was found that deformed fish had higher calcium levels in plasma than did the normal fish, particularly at 180 days and 300 days (groups 3 and 4). In group 3, calcium levels were 8.69±0.28 mg/dL in normal, 10.23±0.36 mg/dL in deformed fish of E. akaara and in group 4, calcium levels were 9.69±0.37 mg/dL in normal, 10.71±0.19 mg/dL in deformed fish respectively. In both groups, calcium levels in deformity group were significantly higher than those in the normal (P<0.05). On the other hand, in group 2 (at 140 days) calcium concentrations of the grouper with deformities was 11.43±0.41 mg/dL and the normal was 13.42±1.52 mg/dl. There were no differences in deformity and normal (P>0.05). At 110 days, size of the fish was too small to collect blood, so we could not proceed.
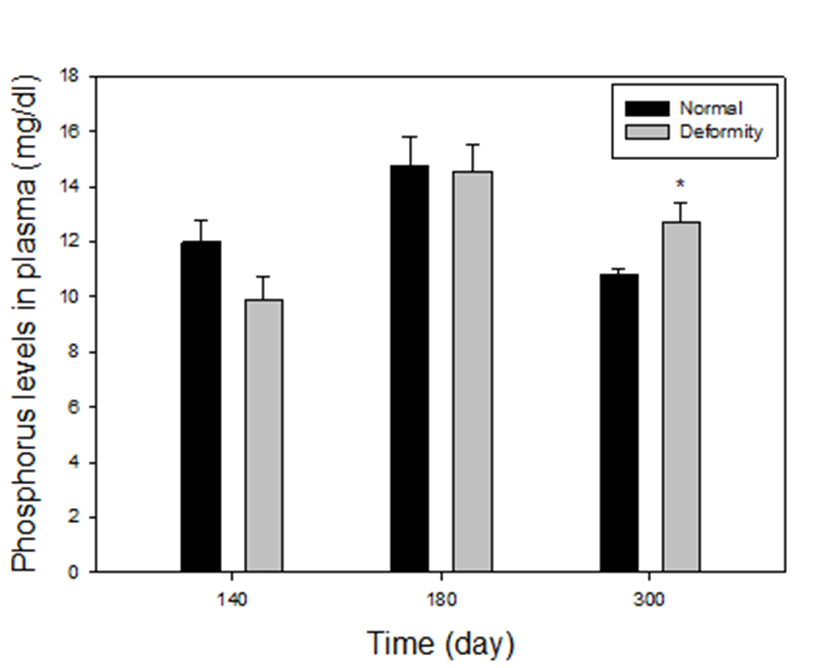
Plasma phosphorus levels between normal and deformed fish at 140, 180 and 300 days after hatching, are presented in Fig. 3. Compared to the normal E. akaara, the deformed fish had higher values in phosphorus at group 4. Phosphorus levels were 10.81±0.19 mg/dL in the normal, 12.70± 0.71 mg/dL in abnormal. The only level of phosphorus in deformity was significantly higher than normal at 300 days (P<0.05). However, in group 2 and 3, phosphorus levels of fish with deformity were 9.86±0.88, 14.53±0.96 mg/dL and normal fish had 12±0.74 mg/dL, 14.75±1.05 mg/dL phosphorus level. There were no statistical differences in normal and deformed fish (P>0.05). At 110 days, size of the fish was too small to collect blood, so we could not proceed.
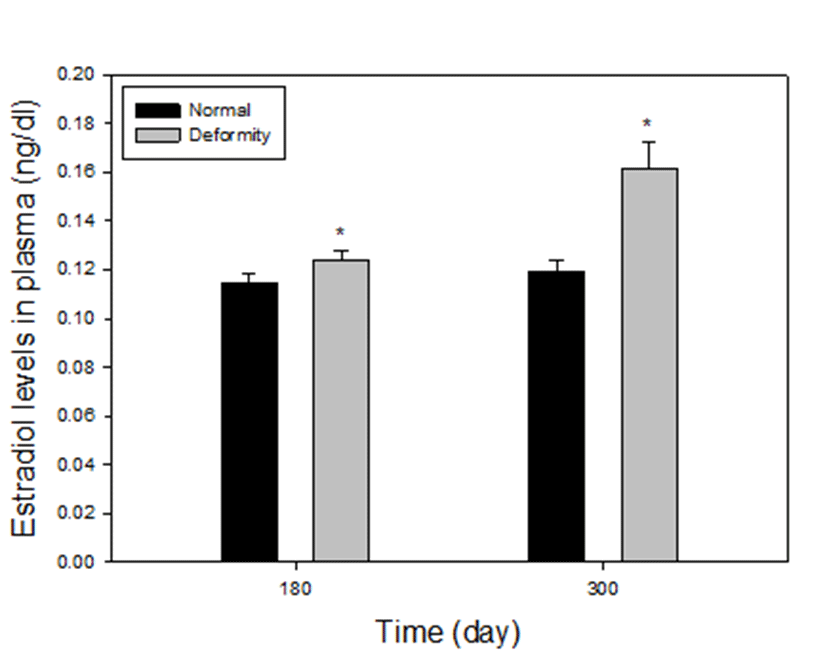
Plasma estradiol-17β levels between normal and deformed fish at 180 and 300 days after hatching, are presented in Fig. 4. The level of estradiol-17β in the deformed fish was statistically higher than those in the normal particularly in the group 3 and 4. In group 3, there were 0.124±0.004 ng/mL and 0.115±0.004 ng/mL of estradiol-17β levels in deformed and normal fish, respectively. At 180 days, the level of estradiol-17β in normal was lower than abnormal individuals (P<0.05). In group 4, the levels of deformity were 0.168±0.011 ng/mL and the normal fish had much lower estradiol-17β level (0.119±0.004 ng/mL) compared to deformed fish, as well (P<0.05). The fish size at 110 days was too small to collect blood and the volume of blood at 140 days was too small to analysis, so we could not proceed.
DISCUSSIONS
One of the most common issues in many cultured fish species is the high rate of body deformities (Barahona-Fernandes, 1982). Common skeletal deformities include curved vertebrae and soft bones in Atlangic salmon (Baeverfjord et al., 1998), deformities with head in frontal bones of common carp (Ogino & Takeda, 1976), and compressed vertebral bodies resulting in scoliosis in haddock (Roy & Lall, 2003) and halibut (Lewis-McCrea & Lall, 2007). In our study, the representative feature of deformation of E. akaara was observed mainly in head, spinal column, operculum and complex mixture.
Lall & Lewis-McCrea (2007) reported that the skeleton in fish undergoes continuous remodeling cycle during their life. In this process, calcium, phosphate, and other ions exchange continuously in blood and extracellular fluids. Our study shows that there were correlation especially between calcium, phosphate and estradiol-17β levels in plasma of normal and deformity in particular developmental stage. At 140 days after hatching of red spotted grouper (group 2), there were similarities of calcium and phosphorus concentrations in blood plasma in normal and deformed groups. However, it was found that calcium, phosphorus and estradiol-17β level in abnormal fish was mostly higher than normal E. akaara at 180 and 300 days (group 3 and 4). According to Lall & Lewis-Mccrea (2007), in bone metabolism of fish, vitamin D3 stimulates absorption of calcium and phosphorus in intestine and kidney. This may also affect the relationship of calcium and phosphorus in blood and bone. If the level of calcium or phosphorus in blood is low, those in bone flow to blood for maintain a constant level, and vice versa. This may cause improper bone formation, abnormalities. Also, according to Gurerreiro et al., (2002), the increase of estradiol-17β level is accompanied by increase of blood plasma calcium and phosphorus. In the present study, calcium and estradiol-17β levels in the deformed fish were higher than those in the normal at 180 and 300 days. The level of phosphorus in the deformity was significantly higher than normal at 300 days. Roy & Lall (2003) observed that phosphorus deficiency is highly associated with bone deformity in haddock. In contrast to the present study, Van Den Avyle et al. (1989) reported that smallmouth bass had similarities of calcium and phosphorus concentrations in blood plasma of deformed and normal fish. However, the metabolism of bone is poorly demonstrated.
The present study suggests that the relationship of the chemical process in bone could be correlated with skeletal deformities from about 180 days in E. akaara. Moreover, it can be appeared that the result; plasma mineral determinations can be useful indicators of metabolic processes that affect bone formation (Van Den Avyle et al., 1989). However, the properties of malformation during various developmental stages are limited by the lack of information. Therefore, more study of skeletal metabolism which is involved in deformities is essential to understand the relationship between skeletal malformations and chemicals correlated with deformity. Further studies to determine the various parameters related skeletal deformity is needed.
