INTRODUCTION
Somatic cell nuclear transfer (SCNT) is a widely used technique with applications in diverse fields, such as animal cloning, biomedical research, and embryonic stem cell production. However, several problems can occur when this technique is used, such as abnormalities in the nuclear reprogramming of SCNT embryos or in offspring phenotypes, as a result of the in vitro environment (Hill et al., 1999). Physical and chemical stresses generated during SCNT can lead to endoplasmic reticulum (ER) stress (Song et al., 2011; Lee et al., 2018), which in turn induces cellular damage, including apoptosis (Szegezdi et al., 2006; Tabas & Ron, 2011; Lee et al., 2018) and mitochondrial dysfunction (Wu et al., 2015). Thus, ER stress is associated with low efficiency levels in SCNT procedures.
ER stress inhibitors are used to reduce ER stress and prevent ER-stress-induced apoptosis. Tauroursodeoxycholicacid (TUDCA), a chemical chaperone derived from bile acid, is an ER stress inhibitor that has been used to stabilize ER protein folding and enhance ER function in a variety of cells and embryos (Xie et al., 2002; Ozcan et al., 2006; Zhang et al., 2012; Basar et al., 2014; Lin et al., 2016). Salubrinal, a eukaryotic initiation factor 2α (eIF2α) dephosphorylating inhibitor, is also used as an ER stress inhibitor (Boyce et al., 2005). Salubrinal promotes the disassembly of the growth arrest and DNA damage-inducible protein 34 (GADD34)–protein phosphatase 1 (PP1) complex, and attenuates ER stress by maintaining eIF2α phosphorylation. Salubrinal is reported to have low cytotoxicity (Boyce et al., 2005; Liu et al., 2012), but is rarely used for embryonic studies.
Embryonic studies using ER stress inhibitors have largely focused on the in vitro culture (IVC) stage for mitigating ER stress and cellular damage (Song et al., 2014). However, ER stress may occur before the IVC stage, such as during the micromanipulation process, and the resulting cellular damage could activate other stress signaling pathways (Lee et al., 2018). Thus, ER stress in SCNT embryos should be mitigated during the micromanipulation stage or immediately after the generation of ER stress instead of during the IVC stage. In this study, we examined the effects of ER stress inhibitor treatment during micromanipulation of porcine oocytes on ER stress, cellular damage, and in vitro development of SCNT embryos.
MATERIALS AND METHODS
All chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) unless otherwise indicated. Tunicamycin (TM), TUDCA (Merck, Darmstadt, Germany), and salubrinal were dissolved in dimethyl sulfoxide (Junsei Chemical, Tokyo, Japan) to prepare stock solutions that were then stored at −20℃ before use.
Previously frozen and thawed porcine skin fibroblasts (pSFs) were seeded in 24-well plates (BD Sciences, San Diego, CA, USA) at a density of 2×104 cells/mL for estimating the appropriate concentrations of the ER stress inhibitors. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; GenDEPOT, Katy, TX, USA) and 1% (v/v) penicillin and streptomycin (P/S; Mediatech, Manassas, VA, USA) at 39℃ and 5% CO2 air concentration. The culture medium was replaced with fresh medium every 2 days. At 80% cell confluency, cells were cultured in the same medium with 2 μg/mL of TM, an ER stress inducer. We added various concentrations of salubrinal (100, 200, or 400 nM) and TUDCA (50, 100, or 200 μM) to two groups of TM-treated cells as well. All cells were then cultured for 3 hr. Non-treated cells served as the control. Cells were trypsinized and lysed in 30 μL Lysis/Binding (L/B) buffer from the Dynabeads(r) mRNA DirectTM kit (Life Technologies, Oslo, Norway). The lysed cells were immediately stored at −75℃ for the analyses of ER stress and apoptosis.
Porcine ovaries were obtained from a local slaughterhouse and transported to the laboratory. Cumulus-oocyte complexes (COCs) were aspirated from antral follicles with a diameter of 3×6 mm using a 10-mL syringe with an 18-gauge needle. COCs were washed in a Tyrode's lactate–4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (TL–HEPES) buffer containing 0.1% (w/v) polyvinyl alcohol (PVA). COCs with homogeneous ooplasm and more than four layers of cumulus cells were selected and cultured in in vitro maturation (IVM) medium at 39℃ and 5% CO2 air concentration for 42–44 hr. The IVM medium consisted of tissue culture medium 199 (TCM199; Gibco) supplemented with 0.1% (w/v) PVA, 3.05 mM D-glucose, 0.91 mM sodium pyruvate, 75 μg/mL penicillin G, 50 μg/mL streptomycin, 0.57 mM cysteine, 10 ng/mL epidermal growth factor, 0.01 IU/mL luteinizing hormone, and 0.01 IU/mL follicle stimulating hormone.
Using four-well dishes, pSFs were seeded and cultured with DMEM supplemented with 15% FBS and 1% (v/v) P/S at 39℃ and 5% CO2 air concentration for 6–7 days, until cells reached 100% confluency, to synchronize the cell cycle at the G0/G1 phase. The culture medium was replaced with fresh medium every 2 days. Cells were trypsinized with 0.05% (w/v) trypsin-ethylenediaminetetraacetic acid (EDTA) and centrifuged (500×g, 5 min, room temperature) in HEPES-buffered TCM199 supplemented with 0.78 mM sodium bicarbonate, 0.14 mM penicillin G, 0.08 mM streptomycin, and 3 mg/mL bovine serum albumin (BSA). Donor cells were placed in the same medium prior to SCNT.
The cumulus cells of matured COCs were removed by vortexing for 3 min in phosphate-buffered saline (PBS; Gibco) supplemented with 0.1% (w/v) hyaluronidase and 0.1% (w/v) polyvinyl pyrolidone (PVP). SCNT was carried out in a micromanipulation medium of HEPES-buffered TCM-BSA containing 5 μg/mL cytochalasin B. Oocyte enucleation was conducted using an injection pipette by removing the first polar body and metaphase II chromosome mass together with a small amount of surrounding ooplasm. Then, a donor cell was injected into the perivitelline space of each enucleated oocyte. Each reconstructed oocyte was helded in a 50-μL droplet of porcine zygote medium-3 (PZM-3; holding medium) until fusion treatment.
The reconstructed oocytes were manually aligned between two wire electrodes (1 mm apart) of a fusion chamber, and covered with a 0.3-M mannitol solution containing 0.1 mM magnesium sulfate, 0.05 mM calcium chloride, and 0.5 mM HEPES (Duchefa Biochemie, Haarlem, Netherlands). For electrofusion and activation, two pulses of direct current at 1.25 kV were applied for 30 μsec using the BTX Electro Cell Manipulator 200 (BTX, San Diego, CA, USA). After fusion and activation, reconstructed oocytes were incubated in holding medium at 39℃ and 5% CO2 air concentration. Fusion state was checked 40 min after treatment.
We investigated three ER stress inhibitor treatments. For the first two treatments, 200 nM salubrinal or 100 μM TUDCA was added to the micromanipulation medium and the holding medium. For the third treatment, both salubrinal and TUDCA (Sal+TUD) were added. After fusion and activation, reconstructed oocytes were incubated in PZM-3 with ER stress inhibitors for an additional 3 hr at 39℃ and 5% CO2 air concentration before IVC.
Treatment and control SCNT embryos were cultured in fresh PZM-3 droplets for 6 days at 39℃ and 5% CO2 air concentration. Embryos were sampled at 20 hr (one-cell stage) and day 6 (blastocyst stage) after fusion and activation, and washed in PBS containing 0.3% (w/v) PVP. Washed embryos were lysed in 30 μL of L/B buffer, and stored immediately at −75℃ for the analyses of ER stress and apoptosis. Some blastocysts were stained with 2 μg/mL Hoechst 33342 for 30 min. Stained embryos were mounted on a glass slide with VECTASHIELD (Vector Laboratories, Burlingame, CA, USA) under a cover slip, and cell numbers were counted using fluorescence microscopy (BX50; Olympus, Tokyo, Japan).
Total pSF RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). The extracted RNA was precipitated using isopropyl alcohol (Junsei Chemical) and resolved with 10 μL of diethyl pyrocarbonate-treated water (Biotech, Markham, Canada). The isolation of poly (A) mRNA from the SCNT embryos was conducted using a Dynabeads(r) mRNA DirectTM kit according to the manufacturer's protocol. Briefly, cryopreserved embryos were thawed and mixed with 30 μL of Dynabeads(r) Oligo (dT)25 by gentle shaking for 8 min at room temperature to allow the poly (A) mRNA tails to hybridize to the oligo (dT)25 on the beads. The beads with attached mRNA were first washed with 100 μL of Washing Buffer A and then 100 μLof Washing Buffer B. A DynaMagTM-Spin Magnet (Invitrogen) was used to separate the beads from the supernatant. Poly (A) mRNA was eluted from the beads by incubation with 12.5 μL of 10 mM tris-hydrochloride buffer at 70℃ for 5 min, and the sample tube was then immediately placed on the magnet. The supernatant, which now contained the mRNA, was transferred to a 0.2-mL microtube on ice. Total RNA and mRNA were reverse-transcribed into cDNA for subsequent analyses. cDNA synthesis was conducted using ReverTra Ace(r) qPCR RT Master Mix (Toyobo, Osaka, Japan) according to the manufacturer's protocol. Each template consisted of 6 μL of total RNA or mRNA. The reaction was carried out in a Veriti(r) 96-well Thermal Cycler (Applied Biosystems, Foster City, CA, USA) at 65℃ for 5 min, 4℃ for 5 min, 37℃ for 20 min, 50℃ for 5 min, and 98℃ for 5 min. To eliminate genomic DNA, 2 μL of 4× DN Master Mix was added to the reaction tube 5 min after the reaction start. Ten minutes later, 2 μL of 5× RT Master Mix II was added for the reverse transcription process. The cDNA products were stored at 4℃.
RT-PCR was used to detect levels of spliced X-box binding protein 1 (Xbp1), a key transcription factor associated with ER stress. Additionally, RT-qPCR was used to measure the expression of mRNAs from ER-stress-associated genes coding for C/EBP homologous protein (CHOP), binding protein (BiP), activating transcription factor 4 (ATF4), and glucose-regulated protein 94 (GRP94), as well as apoptotic genes coding for Bcl2-associated Xprotein (Bax) and caspase-3. For the RT-PCR, cDNA samples were reverse-transcribed using AccuPower(r) Taq PCR PreMix (Bioneer, Daejeon, Korea) according to the manufacturer's instructions. The reaction consisted of an initial denaturation step of 1 min at 72℃, followed by 34 amplification cycles of denaturation at 95℃ for 30 sec, annealing at 58℃ for 30 sec, and extension at 72℃ for 50 sec. A final extension step of 5 min at 72℃ was performed to complete the reaction. PCR products were examined with ultraviolet irradiation on a 4% agarose gel (Amresco, Cleveland, OH, USA) stained with 0.05% ethidium bromide (Bioneer). Band intensity was measured densitometrically using Gel DocTM XR+ (Bio-Rad, Berkeley, CA, USA).RT-qPCR was conducted using Power SYBR Green PCR Master Mix (TOPrealTM qPCR 2X PreMIX, SYBR Green with high ROX; Enzynomics, Daejeon, Korea) in a StepOne Plus system (Applied Biosystems). The comparative CT (∆∆CT) method (Livak & Schmittgen, 2001) was used to measure the relative levels of mRNA expressed by each target gene. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize target gene expression. The primer pairs synthesized for each gene are listed in Table 1.
At least three replicates were used for each statistical test. RT-PCR and RT-qPCR data were compared using analysis of variance and Duncan's multiple range test. Developmental rates were analyzed using the chi-square test, and blastocyst cell numbers were compared using Student's t-test. All analyses were conducted using the Statistical Analysis System software package (v.9.3; SAS Institute, Cary, NC, USA).
RESULTS
Addition of TUDCA significantly reduced (p<0.05) the levels of Xbp1 splicing, which was induced by TM, in porcine fibroblasts in all TUDCA treatment groups (Fig. 1A). Expression levels of the ER-stress-associated genes (BiP,GRP94, ATF4, and CHOP) also decreased with addition of 100 μM and 200 μM TUDCA, but these differences were not significant (Fig. 1B). Expression levels of the ER-stress-associated genes were similar in the 100 μM- and 200 μM-TUDCA treatments.
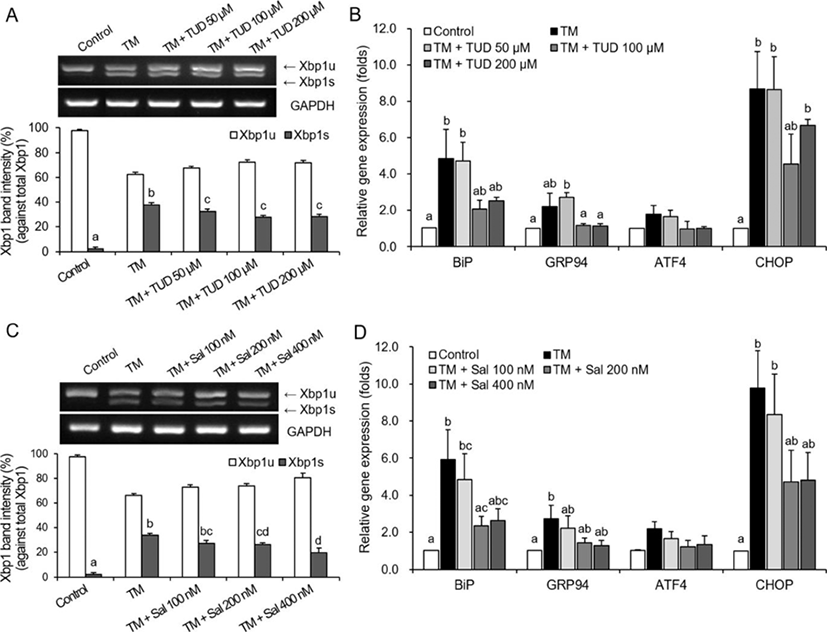
The effects of using 100 μM and 200 μM TUDCA on the in vitro development of porcine parthenogenetic embryos were examined in an additional experiment. As no differences in development were observed between treatments using the two TUDCA concentrations (unpublished data), 100 μM TUDCA was used for the SCNT experiments.
The addition of both 200 nM and 400 nM salubrinal also significantly reduced (p<0.05) levels of TM-induced Xbp1 splicing in porcine fibroblasts (Fig. 1C). Similarly, salubrinal resulted in down-regulation of ER-stress-associated genes. Overall, gene transcription levels were lower in treatments with 200 nM and 400 nM salubrinal compared to levels in the TM-only treatment (Fig. 1D). However, these decreases were not significantly different, except for BiP in the 200-nM salubrinal treatment (Fig. 1D).
The effects of using 200 nM and 400 nM salubrinal on the in vitro development of porcine parthenogenetic embryos were also examined in an additional experiment. The results revealed that addition of 400 nM salubrinal caused degeneration of parthenogenetic embryos (unpublished data). Thus, we used 200 nM salubrinal for the SCNT experiments.
At the one-cell stage, a significant decrease in Xbp1 splicing levels in the SCNT embryos was observed only in the TUDCA treatment (Fig. 2A). The transcription levels of all ER-stress-associated genes decreased in all embryos treated with ER stress inhibitors, but transcription levels varied among genes (Fig. 2B). The transcription levels of GRP94, ATF4, and CHOP decreased significantly when SCNT embryos were treated with TUDCA (p<0.05). In the Sal+TUD treatment, the transcription levels of BiP and GRP94 were significantly lower (p<0.05) than levels in the control, and in the salubrinal treatment only CHOP had a significantly lower transcription level (p<0.05) than control levels.
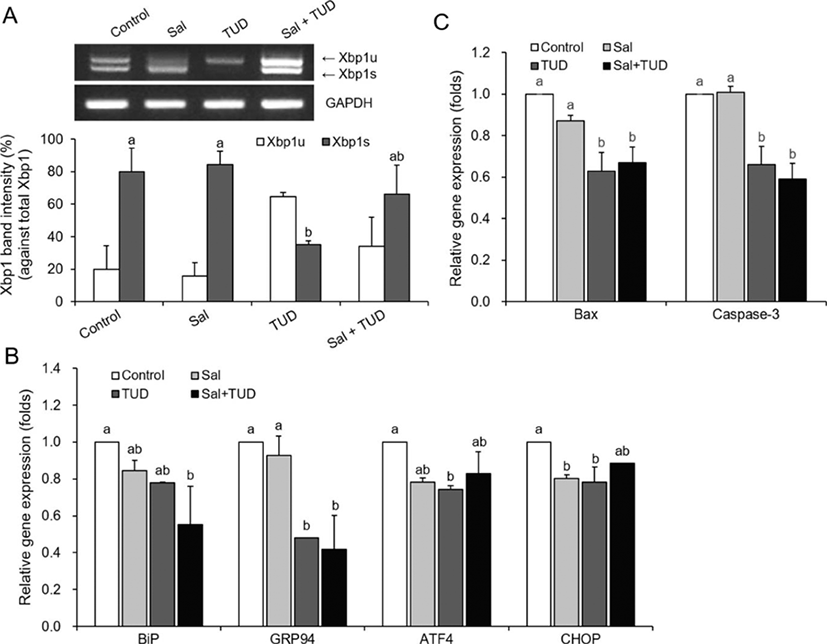
During micromanipulation, addition of salubrinal did not reduce apoptosis rates in the SCNT embryos at the one-cell stage. However, transcription levels of Bax and caspase-3mRNAs were significantly lower (p<0.05) in the TUDCA and Sal+TUD treatments compared with control levels (Fig. 2C).
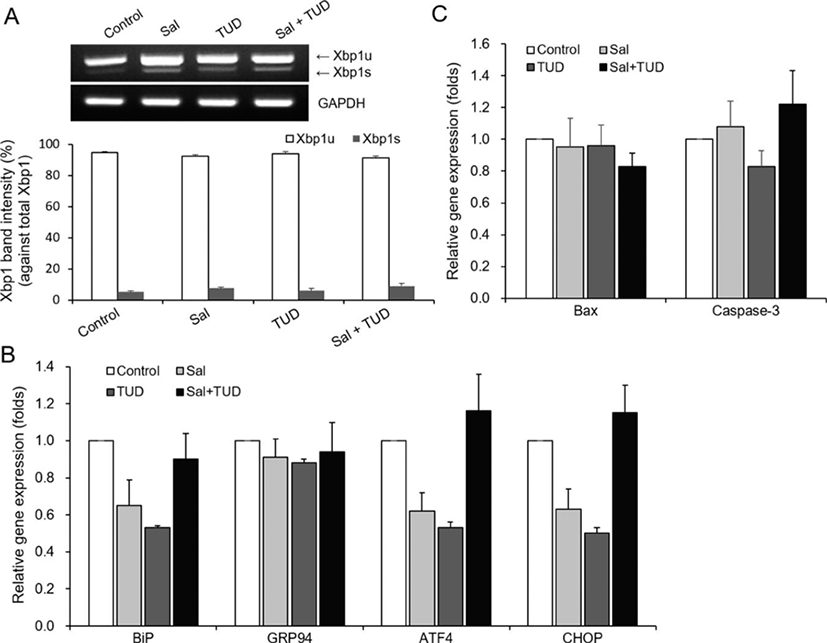
No significant differences were observed for levels of Xbp1 splicing or ER-stress-associated gene expression among all treatments and the control in SCNT embryos at the blastocyst stage (Fig. 3A, B), and no significant differences in the transcription levels of the Bax and caspase-3 apoptotic genes were observed among groups (Fig. 3C).
No differences in cleavage rate (69.2%–73.9%) were observed between control and treatment groups. Blastocyst formation rates and mean blastocyst cell numbers in the salubrinal (15.4% and 53.7±4.5, respectively) and Sal+TUD (12.2% and 54.0±3.0, respectively) treatments did not differ significantly from those of control embryos (12.6% and 41.7±3.1, respectively). However, blastocyst development (20.2%) and mean blastocyst cell number (63.0±7.2) were significantly higher (p<0.05) in embryos treated with TUDCA than in control embryos (Table 2).
Experiments were repeated 6 times in each treatment group.
Sal, salubrinal; TUD, tauroursodeoxycholic acid (TUDCA).
DISCUSSION
In the in vitro environment, stimuli from experimental processes can cause ER stress in embryos. Both electric stimuli and the calcium (Ca2+)ionophore (A23187), which are commonly used in SCNT, have been reported to disturb Ca2+ homeostasis of the ER and induce ER stress (Brostrom et al., 1995; Fernandez et al., 1996). Our previous study revealed that A23187-mediated activation increased levels of ER stress and apoptosis in porcine parthenogenetic embryos (Park et al., 2017). In a related study, expression levels of ER-stress-associated genes were reported to be higher in SCNT embryos than in in vitro fertilized (IVF) embryos, suggesting that ER stress and ER-stress-induced apoptosis may occur during SCNT (Lee et al., 2018). Micromanipulation has also been reported to generate excessive amounts of reactive oxygen species (ROS) in SCNT embryos (Hwang et al., 2013), and ROS is a trigger of ER stress (Malhotra et al., 2008).
The results from our study indicate that addition of ER stress inhibitors during micromanipulation can reduce ER stress and subsequent embryonic cell damage. However, compared to TUDCA, the addition of salubrinal or both salubrinal and TUDCA (Sal+TUD treatment) did not reduce the levels of Xbp1 splicing or expression of several ER-stress-associated genes. Although combining both salubrinal and TUDCA led to reduced expression of BiP and GRP94, the expression of ATF4 and CHOP increased relative to treatments in which each inhibitor was used individually (see Fig. 2B). No significant differences in gene expression were observed among all groups at the blastocyst stage. This may have been because the effects of the ER stress inhibitors diminished during the 6 days of IVC.The embryos developed to the blastocyst stage would have already overcome the ER stress induced by micromanipulation, so that there were no differences in levels of Xbp1 splicing and expression of ER-stress-associated genes between treatment and control groups.
In the treatment using 100 μM TUDCA, inhibition of ER stress during micromanipulation, but not while culturing, resulted in better outcomes of in vitro development at the blastocyst stage, similar to results reported in other studies. Addition of 100 μM TUDCA during the IVC of porcine SCNT embryos significantly increased blastocyst formation, cell numbers of the inner cell mass, and total blastocyst cell numbers compared to levels in control embryos (Lin et al., 2016). TUDCA has also been reported to inhibit ER stress and ER-stress-induced apoptosis in porcine parthenogenetic embryos, and promote maturation of COCs and embryonic development (Zhang et al., 2012b). Similarly, Song et al. (2011) reported that addition of TUDCA reduced levels of Xbp1 splicing, transcription of the ER-stress-associated gene GRP78, which codes for ATF6, an α-mannidose-like protein that enhances ER degradation, and blastomere apoptosis in bovine SCNT embryos. In addition, TUDCA can reduce hyperosmolar-induced ER stress and ER-stress-induced apoptosis during oocyte development, and promote embryonic development in mouse embryos (Zhang et al., 2012a; Basar et al., 2014; Mochizuki et al., 2018). In contrast to previous studies, we applied TUDCA only during micromanipulation and 3 h after fusion and activation, not during the IVC stage, to enhance the in vitro development of the SCNT embryos. Our results reveal that immediate suppression of ER stress during micromanipulation can enhance development in SCNT embryos by increasing blastocyst cell numbers. Thus, ER stress has to be inhibited or removed during micromanipulation for optimal reprogramming of SCNT embryos.
Although it is also an ER stress inhibitor, addition of salubrinal did not enhance embryonic development in our study, implying that salubrinal is not as effective as TUDCA. However, addition of 100 nM and 200 nM salubrinal to mouse COCs during IVM was reported to mitigate the deficiencies induced by ER stress, including lowered levels of protein secretion and mitochondrial activity, and lowered developmental capacity of IVF embryos (Wu et al., 2012). Addition of 400 nM salubrinal during IVM has also been shown to promote in vitro development of bovine IVF embryos and reduce ER stress markers (Sutton-McDowall et al., 2016). Inconsistencies between these results and our findings are likely due to the different species used for experiments and differences in treatment conditions. Additionally, different ER stress factors are involved during SCNT and IVM, which may have different effects on the activation of the protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway and the inhibitory function of salubrinal, an eIF2α-specific ER stress inhibitor.
IRE1α, PERK, and ATF6 are all sensors of the unfolded protein response (UPR), but have different sensitivities depending on the cause of ER stress. For example, PERK responds rapidly to ER stress caused by disturbance of Ca2+ concentrations in the ER lumen, but does not respond to ER stress caused by reducing agents (Hetz et al., 2012).
Although apoptotic gene expression decreased in embryos treated with TUDCA, addition of both salubrinal and TUDCA did not enhance embryonic development in our study. This may have been due to increased expression of ATF4 and CHOP at the one-cell stage. Because CHOP is an important mediator of apoptosis induced by ER stress (Zinszneret al., 1998), CHOP-mediated apoptotic pathways may still have affected embryonic development in the Sal+TUD treatment, despite the reduced expression of Bax and caspase-3. Furthermore, levels of Xbp1 splicing decreased in embryos treated with TUDCA, but not in the other embryos. This implies that Xbp1 splicing in the early SCNT embryo is important for in vitro development. Zhang et al. (2012a) reported that peak Xbp1 expression occurred at the four-cell stage, which is the same stage of genomic activation. This implies that Xbp1 may be associated with embryonic development in pigs.
In conclusion, our results reveal that addition of ER stress inhibitors, particularly TUDCA, during micromanipulation reduces ER stress levels in early porcine SCNT embryos, and increases embryonic developmental potential in vitro by preventing apoptosis. Further studies are needed to clarify the relationship between UPR signaling and SCNT embryonic reprogramming.
