INTRODUCTION
Analysis of blood cell can reveal a measurable amount of physiological changes in fish more rapidly than any physiological assessment parameters (Rowan, 2007). It can also respond to changes in other tissues due to exposure of environmental stressors (i.e., temperature, salinity, overcrowding, and water quality) (Ruas et al., 2008). Therefore, examining blood cells has been considered a valuable approach for analyzing the health status of fish and enhancing our understanding of the relationship of blood characteristics with the habitat and adaptability of a species to an environment (Fazio et al., 2012; Shahjahan et al., 2018; De et al., 2019; Islam et al., 2019). Thus, the use of blood indicators in fisheries research is growing rapidly as it is very important in toxicological research, environmental monitoring and predicting of fish health conditions (Barcellos et al., 2004).
Unlike human and most mammals, fish possess an oval-shaped structure of erythrocyte with a condensed nucleus (Jagoe & Welter, 1995). Fish erythrocytes are more responsive to environmental stresses, and often vary in morphology and effectiveness of oxygen transport (Lowe-Jinde & Niimi, 1983). However, variations in erythrocytes and its nuclear size and structure within species are smaller compared to interspecies differences (Najiah et al., 2008; Fang et al., 2014). In our previous study, we examined the blood cell morphology and number of E. akaara under high water temperature exposure and the findings are useful to assess the primary immune response of fish under stressful conditions (i.e., thermal stress) (Rahman et al., 2019).
The red spotted grouper is a sub-tropical species of serranidae family which prefers to inhabit shallower depths of the coastal area (Sadovy & Cornish, 2000). It is a promising aquaculture species due to its high market value in southern Japan, Korea, Hong Kong, Taiwan, and southern China (Morris et al., 2000). The normal range of water temperature in the tropics to which fish are adapted is 25°C–35°C (Howerton, 2001). The optimal water temperature at which E. akaara can live without stress was reported to be 24°C–28°C (Cho et al., 2015; Lee & Baek, 2018). However, temperature can increase to a point that may become harmful for growth and damage physiological processes (Fu et al., 2018). Though a number of studies were conducted to make a better understanding of blood cell morphology of fishes under various environmental toxicants (Rowan, 2007; Osman et al., 2018; Shahjahan et al., 2018; Islam et al., 2019; Rahman et al., 2019), the effect of high temperature on erythrocyte and nuclear morphometry are not well studied. Therefore, the present study was intended to evaluate the morphometric indices of erythrocytes and their nucleus under elevated water temperature which will help to check the health status of E. akaara in a quick time.
MATERIALS AND METHODS
180 healthy and active specimens of E. akaara with an average total length (TL) of 8.28±0.10 cm and body weight (BW) of 8.53±0.27 g were collected from the Marine Science Institute, Jeju National University, Korea. The juveniles were acclimated in aquaria at a water temperature of 25±0.5°C, salinity of 34 psu, dissolved oxygen levels≥6.6 mg/L, pH of 7.8, and a 12L: 12D photoperiod for 2 weeks before the experiment. The fish were fed commercial feed (Otohime Hirame; Marubeni Nisshin Feed Co., Ltd., Tokyo, Japan) twice a day until satiation. The fishes were exposed to four temperature conditions (25°C as control, 28°C, 31°C, and 34°C), each with three replications for 6 weeks. Temperature was gradually increased (Δ1°C/h) using a thermostat (OKE-6422H; OKE, Busan, Korea) to reach the target temperature conditions (25°C, 28°C, 31°C, and 34°C). Approximately 10% of the water was replaced daily with filtered clean seawater as feces and debris were siphoned from the tank.
15 fishes were sampled from each temperature group (i.e., 5 fishes from each aquaria; n=15) on each sampling day (i.e., 2, 7, and 42 days of thermal exposure). Fish were randomly captured and lightly anaesthetized with 300 ppm 2-phenoxyethanol (Sigma Aldrich, St. Louis, MO, USA). Blood was collected from the caudal vein using heparinized capillary tubes, labeled, and stored in 1.5 mL centrifuge tubes. The whole blood was withdrawn less than 1 min per fish to avoid handling stress.
Blood smears were prepared immediately after collection, air-dried for 10 min, fixed in 95% methanol for 5 min, stained with Wright-Giemsa solution, rinsed with distilled water, air-dried and mounted with malinol. Three slides were prepared from each fish and then photos were taken under light microscopy. Erythrocytes in fish are elliptical, and as such, two different diameters are provided: erythrocyte major axis (EL) and erythrocyte minor axis (EW). Nucleus major axis (NL) and nucleus minor axis (NW) were also obtained. On each slide, EL and EW of randomly selected 100 mature erythrocytes and their nuclei (NL and NW) were measured by an Olympus ocular micrometer at a magnification of ×600 (Olympus BX-50, Japan). Erythrocyte and nuclear sizes (ES and NS) were calculated according to formulas [(EL×EW×π)/4] and [(NL×NW×π)/4], respectively (Metin et al., 2008).
Data have been presented as mean±SEM and analyzed by using SPSS statistics software (ver. 21.0; IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test was used to assess the significant differences among the different temperature groups. Pearson’s correlation coefficient test was conducted to estimate the relationships among the morphometric indices of erythrocyte. In addition, regression analysis and goodness of fit (R2) were determined when there was a significant correlation between tested variables. The results were considered significant at p<0.05.
RESULTS AND DISCUSSION
Elevated water temperature induced significant changes in erythrocytes and nucleus structure of 31°C and 34°C groups, compared to those in the 25°C and 28°C groups. Fishes of 31°C and 34°C groups showed round-shaped erythrocyte structure containing large nucleus, whereas erythrocytes of 25°C and 28°C groups showed oval-shaped structure with condensed nucleus (Fig. 1).
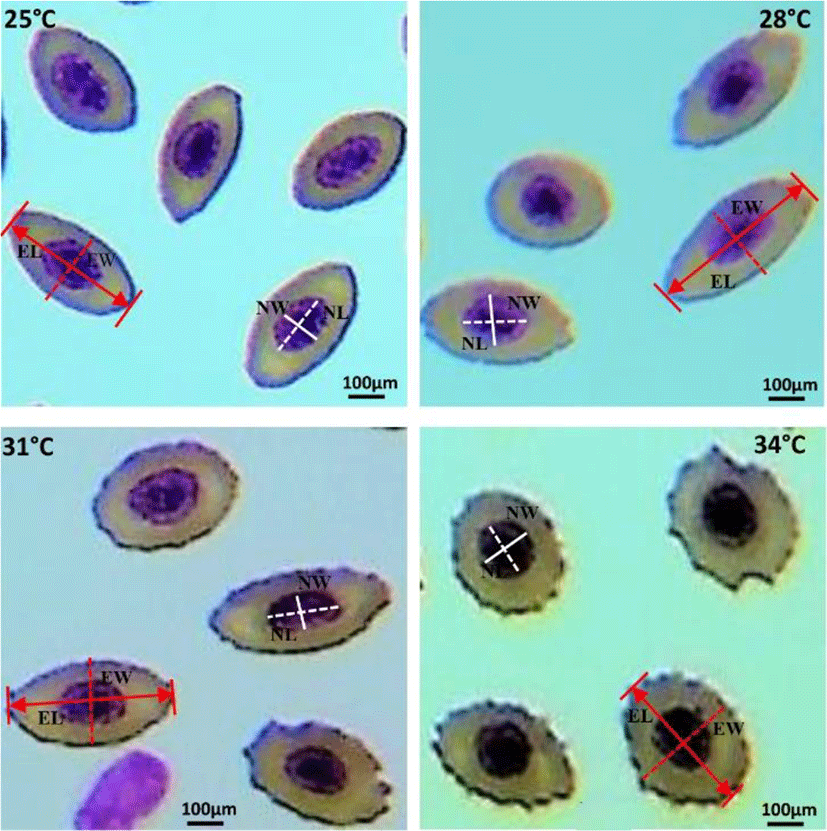
The morphometric indices of erythrocytes and their nucleus under different water temperatures (25°C, 28°C, 31°C, and 34°C) are shown in Table 1. Major and minor axis morphometry, and size of erythrocytes and nucleus differed significantly during the thermal exposure period (42 days). The major and minor axis of erythrocytes and nucleus were changed reversely. EL and NL were significantly (p<0.05) decreased, whereas EW and NW were increased at higher water temperature (31°C and 34°C). In addition, the major-minor axis proportion of erythrocytes and their nucleus (EL/EW; NL/NW) were decreased with increasing water temperature (31°C and 34°C). On the other hand, the size of erythrocytes and their nucleus (ES, NS and ES/NS) showed irregular changes under elevated water temperature.
Sampling was done at three different time points (2, 7, and 42 days). Values are mean±SEM of three replicates (n=15; 5 fish per tank). 100 erythrocytes and their nucleus were measured from each blood smeared slide.
Different lowercase letters indicate the significant difference between groups at equivalent times (ANOVA, Duncan’s multiple range test; p<0.05).
EL, erythrocyte major axis; EW, erythrocyte minor axis; NL, nucleus major axis; NW, nucleus minor axis; ES, erythrocyte size; NS, nucleus size.
Both positive and negative correlations were observed among the morphometric indices of erythrocytes and nucleus of red spotted grouper (Table 2 and Fig. 2a–f). A highly significant positive relationship was found in EL vs. NL and EW vs. NW, whereas significant negative relationship was detected in EL vs. EW, NL vs. NW, EL vs. NW, and EW vs. NL.
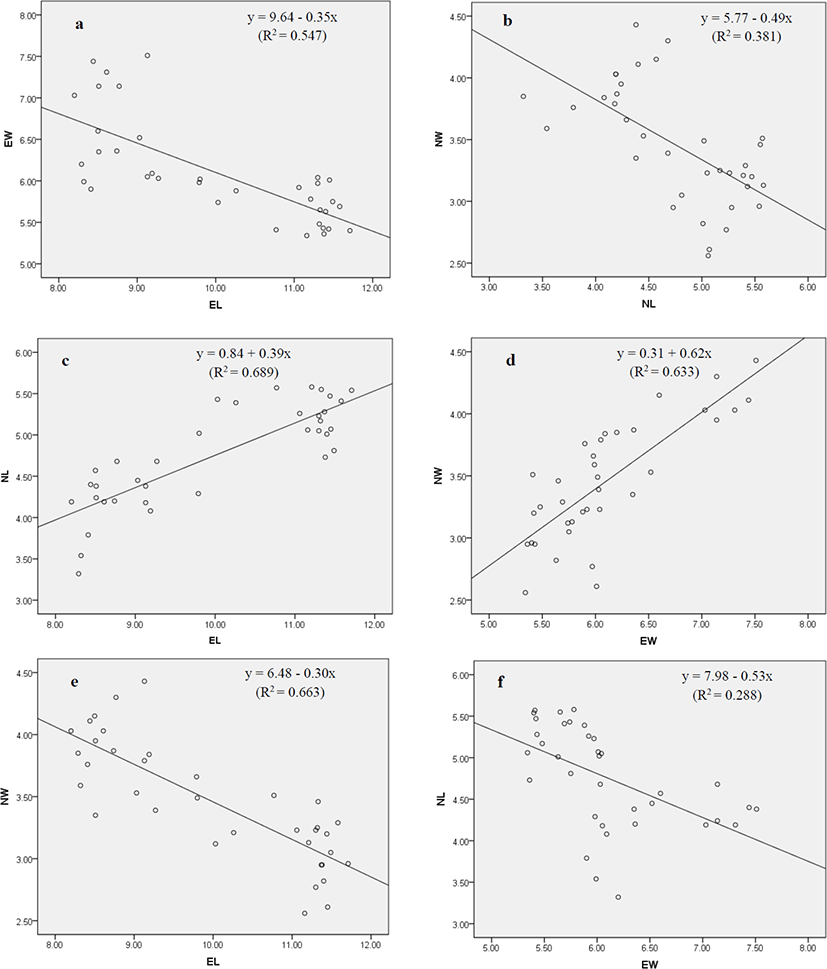
See the regression plot (Fig. 2a-f) for each paired samples **p<0.01.
EL, erythrocyte major axis; EW, erythrocyte minor axis; NL, nucleus major axis; NW, nucleus minor axis; ES, erythrocyte size; NS, nucleus size.
Since erythrocytes are influenced by a variety of environmental stressors, they have the potential to be used as stress indicators. Their evaluation in fish has become an important means of understanding the toxicological impacts of exposure hazards (Borges et al., 2007). In this study, morphometric indices of erythrocytes and their nucleus were evaluated under different water temperatures (25°C, 28°C, 31°C, and 34°C) to investigate their use as an indicator of thermal stress in red spotted grouper.
In the present study, changes in erythrocytes and their nucleus structure were observed in E. akaara under elevated water temperature (31°C and 34°C). In our previous study, we reported that thermal stress causes various types of erythrocytic cellular abnormalities (ECA) in E. akaara (Rahman et al., 2019). The erythrocytes of 25°C and 28°C were found in oval to elliptical shape, but turned into round shape when exposed to 31°C and 34°C. These altered structure in erythrocytes and nucleus can induce anemic condition to the thermally stressed fishes. Similar abnormalities in erythrocyte and nuclei were reported in striped catfish, Pangasianodon hypophthalmus when treated with higher temperature (36°C) for 7–28 days (Shahjahan et al., 2018; Islam et al., 2019). The observed structural changes in erythrocyte and nuclei may be attributed to one or more of the following factors: (i) unequal distribution of hemoglobin (Mekkawy et al., 2011); (ii) necrosis of the red blood cells (Ateeq et al., 2002); (iii) interruption in lipid solubility to erythrocyte’s membrane (Walia et al., 2013).
Fish erythrocytes and their nucleus have a wide range of shape and sizes among different species (Jagoe & Welter, 1995). The changes in erythrocytes depend on the fish species, age, health and the environment where the fish live (Vazquez & Guerrero, 2007; Zexia et al., 2007; Motlagh et al., 2012). Measurement of morphometric indices of erythrocytes and nucleus [major axis, minor axis, and size] under different water temperatures (25°C, 28°C, 31°C, and 34°C) demonstrated that erythrocytes and their nucleus varied in size at higher temperature (31°C and 34°C) which is the clear indication of stress. The observed decrease in major axis (EL, NL) and increase in minor axis (EW, NW) of erythrocytes and their nucleus might be because of changing the erythrocyte’s shape from elongate to round which causes the reduction in major axis and enlargement in minor axis. The observed variation could also be explained by the following factors: (i) differing the dissolved oxygen levels in experimental groups; (ii) the maturation stage of the erythrocytes; (iii) the sample size of research. These findings are consistent with those of Rowan (2007), who reported that erythrocyte and nucleus morphometry of brown bullheads (Ameiurus nebulosus) were affected due to the pollution of river water by agricultural runoffs or industrial effluents. Similar results were also reported in Nile tilapia (Oreochromis niloticus niloticus) and African catfish (Clarias gariepinus) collected from contaminated sites of the river Nile (Osman et al., 2018). In this study, the strong relationships were observed among the morphometric indices of erythrocytes and their nucleus. Hardie & Hebert (2003) reported that erythrocytes and nuclear sizes are significantly correlated in ray-finned fishes, cartilaginous fishes and all species combined.
In conclusion, the results of the present study reveal that elevated water temperature (31°C and 34°C) beyond the optimal range (24°C–28°C) affects the morphometric indices of erythrocytes and their nucleus, especially EL, EW, NL, and NW which can be considered as stress indicators (i.e., thermal stress) of red spotted grouper. This fine information may be helpful to monitor the health status of E. akaara and probably for other fish species.
