INTRODUCTION
The first phase of the ecological risk assessment procedure due to environmental factors is hazard identification (NRC, 1983). Among the biomarkers used for assessing ecotoxicity, reproductive biomarkers are an integral part of assessing long-term and continuous effects from environmental factors (Huggett et al., 1992; Schmitt et al., 2005). Histological biomarkers, including sex, stages of gonadal development, tumors, intersex, and atresia, are also an essential component for researching the reproductive health of fishes (Blazer, 2002; Kokokiris et al., 2005; Lee et al., 2010; Colin et al., 2016).
The rock bream, Oplegnathus fasciatus, is a teleost that belongs to Perciformes and Oplegnathidae. It is a coastal fish species that inhabits coastal waters of the South China Sea, Japan, and Korea (Kim et al., 2005). In Korea, the rock bream is a very important species for aquaculture and conservation of fishery resources. The rock bream is produced artificially from the hatched larvae to the early stage before the juveniles are grown to approximately 5.0 cm in total length and ready to be released to the wild. Nearly 9 million fishes were released to the wild from 2012 to 2015 (FIRA, 2015). The present study aimed to investigate the gonad structural abnormality and intersexuality of the rock bream, O. fasciatus, inhabiting the southern coast of Korea, measured during a survey of their reproductive potential.
MATERIALS & METHODS
In total, 448 rock bream were used in the analysis with total length of 27.5±6.8 cm and total weight of 522.9±385.6 g. The samples were collected using stow nets and trawl nets in waters near Sacheon, Mokpo, and Yeosu of Korea (Fig. 1).
Five preparations of 1 cm2 area were analyzed from each individual after categorizing the gonads by fore, middle, and rear parts using the methods described by Lee et al. (2010). Each sample was fixed with 10% neutral buffered formalin and then dehydrated through an ethanol series (70%–100%). The tissues were embedded in paraplast (McCormick Scientific, St. Louis, MO). Embedded tissues were serially sectioned to a thickness of 4–6 µm using a microtome (RM2235, Leica, Germany). Samples were stained with Mayer’s hematoxylin–0.5% eosin (H-E) stain and Masson’s trichrome stain.
RESULTS AND DISCUSSION
The sex ratio of O. fasciatus was 1:0.46, with 307 female specimens and 141 male specimens. Females made up approximately 68.5% of the entire sample, showing a significantly higher composition than that of the males (p<0.05; Table 1).
| Specimen number | Sax ratio | Chi-square | p-value | |||
|---|---|---|---|---|---|---|
| Total | Female | Male | F:M | F/F+M (%) | ||
| 448 | 307 | 141 | 1:0.46 | 68.5 | 61.509 | 0.000 |
As oocyte atresia proceeds, the shape of the oocytes becomes irregular. We observed shrinking of the zona radiata and more eosinophilic reactions than those in the normal oocytes (Fig. 2A and B). Moreover, we identified the granulation of chromatin and the collapse of the nuclear membrane. A loss of eosinophilic yolk granules was observed in the cytoplasm. The yolk granules in the cortex and medulla were homogeneous, with the medulla found to be more basophilic in the H-E stain than the cortex part (Fig. 2B and C). Oocyte atresia occurred in 85.3% of samples (Table 2).
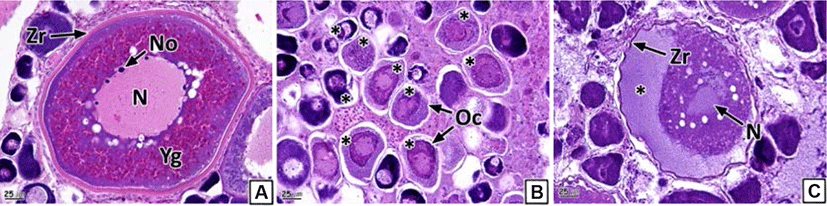
We could not morphologically identify the neoplasm in the form of nodules or tumors from the ovaries. However, histological analysis showed a cluster of multiple oocytes in different stages of development and a newly formed connective tissue layer outside the ovarian membrane (Fig. 3). The newly formed connective tissue layer turned blue in the Masson’s trichrome stain (Fig. 3C). Oocytes and fatty tissues showed eosinophilic stainability and vacuoles in the H-E stain, respectively (Fig. 3B). The prevalence of ovarian neoplasm recorded was 21.5% (Table 2).
Oocyte atresia is a normal phenomenon during the degeneration of oocytes after spawning in teleosts (Genten et al., 2009). Oocyte atresia has been reported as an endocrine disorder of female aquatic animals, which is inflicted on oogenesis owing to various environmental factors. The species currently affected include the common carp (Blazer, 2002); sole, Solea solea (Cuevas et al., 2015); brown bullhead, Ameiurus nebulosus; smallmouth bass, Micropterus dolomieu; yellow perch, Perca flavescens (Pinkney et al., 2017) and Mytilus trossulus (Smolarz et al., 2017). The histological properties seen in the rock bream during the present study were similar to those from these previous studies.
Gonadal neoplasm of aquatic animals has been mainly reported in the form of nodules, tumors, or mixed germ cell-stromal neoplasia from crucian carp, Carassius carassius (Fregeneda-Grandes et al., 2010); softshell clam, Mya arenaria (Barber, 1996) and mussel, Mytilus galloprovincialis (Carballal et al., 2015). These previous studies focused on various chemical pollutants as the cause of these anomalies. However, Spitsbergen et al. (2012) stated that diet and the aquaculture environment were the primary causes of confirmed neoplasia in multiple organs, including gonads, from zebra fishes raised in an aquarium.
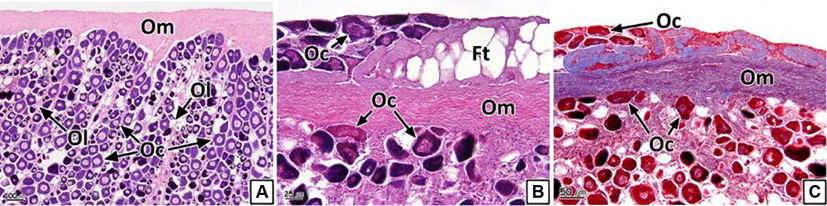
Each testicular lobule and cyst comprising the testis were divided by a thin membrane of connective tissues (Fig. 4A and B). However, part of or the entire membranes of the testicular lobules and cysts of some individuals were disintegrated; thus, we could not discern the form of the individual testicular lobules and cysts (Fig. 4C and D). The prevalence of collapsed testicular lobules and cysts was 73.1% (Table 2).
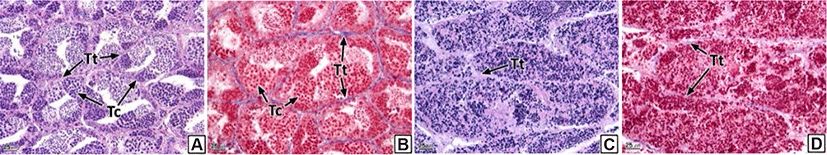
Similar to the ovaries, anatomical neoplasia was not confirmed in the testes. However, in histological analysis, neoplasia was observed in the form of groupings of basophilic sperm combined with connective tissues in the outside of the testicular outer membrane (Fig. 5). Testicular neoplasm was prevalent in 37.6% of the male samples (Table 2).
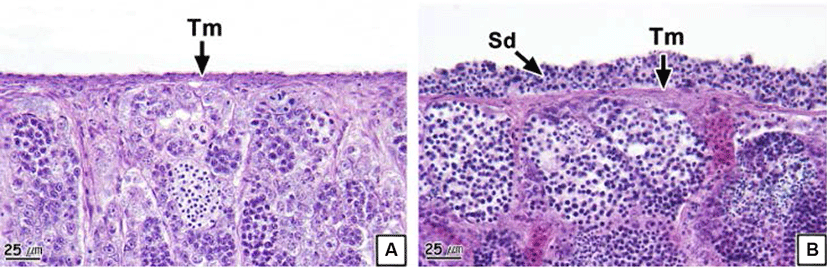
The testis of teleosts consist of multiple testicular lobules surrounded by thin connective tissues (Lee & Huh, 2000; Blazer, 2002). Each testicular lobule retains numerous testicular cysts, and germ cells develop synchronously in the same testicular cyst (Lee et al., 2010). Spermatogenesis in teleosts is controlled by the central nervous system and endocrinological function of Leydig cells and Sertoli cells spread around the testis (Grier, 1981). Therefore, the deformation of testicular lobules and cysts in the rock bream could be determined to be histological evidence of an inability to obtain normal spermatogenesis.
We also observed the formation of scales inside the gonadal cavity in some individual samples (Fig. 6). The scales showed eosinophilic stainability in the H-E stain and the center of the scales turned red and the peripheral parts stained to blue in Masson's trichrome stain (Fig. 6B and C). The prevalence of scale formation in the ovary and testis was 2.0% and 2.1% respectively (Table 2).
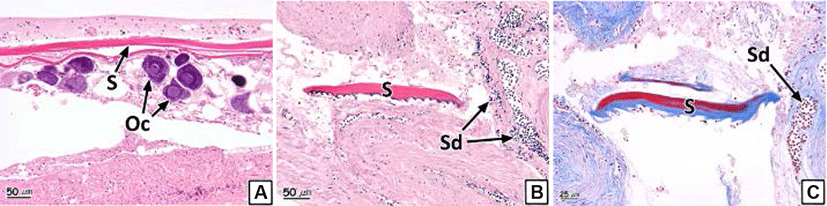
Scales of teleosts are formed by the hardening of connective tissues in the dermis of the skin (Takashima & Hibiya, 1995); moreover, vital somatic tissues inside the gonads also consist of connective tissues (Genten et al., 2009). Chemical factors such as EDC’s (endocrine disrupting chemicals) affects influence adipogenesis and osteogenesis in mesenchymal stem cells (Bateman et al., 2017). Chemical factors influence the synthesis of collagen, a major component of connective tissue, in fish such as Salmo gairdneri, Salvelinus fontinalis, Pimephales promrlas, Ictalurus punctatus (Mayer et al., 1977) and Pimephales promelas (Mehrle et al., 1981). In addition, in association with other gonad abnormalities identified in this study, one of the important causes of scale formation in the gonads is considered to be chemicals. Although the formation of scales inside the gonads in rock bream resulted from the disruption of the endocrine system due to chemical factors during the early embryonic stage, especially when connective tissues are differentiated, additional research regarding this topic is required.
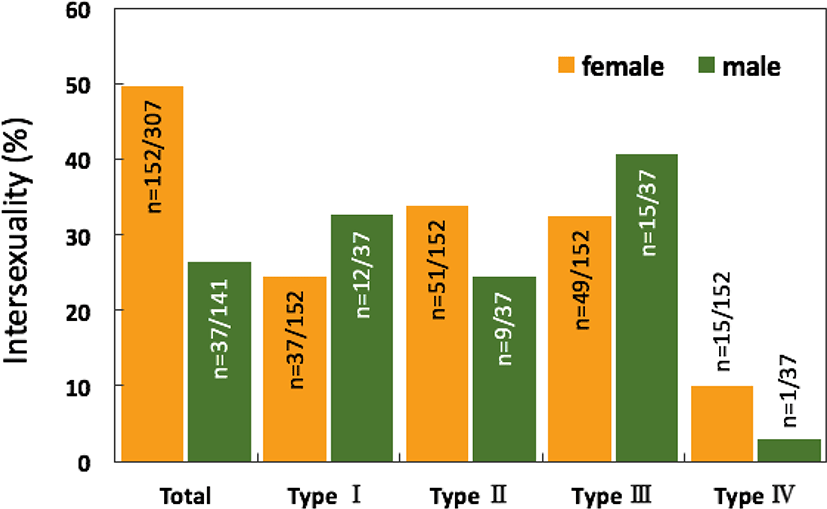
The overall intersexuality in the rock breams reached 42.2%, being higher in the female samples (49.5% in females and 26.2% in males; Fig. 7).
The intersex type observed in the gonads was histologically classified into four types (I, II, III, and IV). Type I shows germ cells of the opposite sex spread throughout the connective tissues of the gonadal cavity (Figs. 8A and 9A). Type II is the distribution of germ cells of the opposite sex in the newly formed connective tissues outside the gonad outer membrane (Figs. 8B and 9B). Type III represents the form in which the sperm and oocytes coexist in the connective tissues outside the gonad outer membrane (Figs. 8C and 9C). Type IV is the form in which the sperm and oocytes coexist in connective tissues outside the gonad outer membrane as well as in the gonadal cavity (Figs. 8D and 9D).
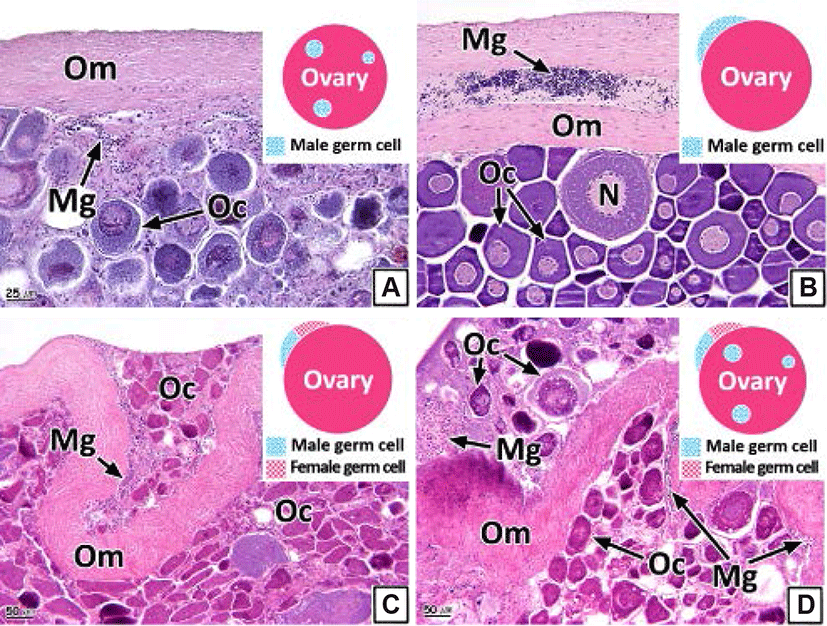
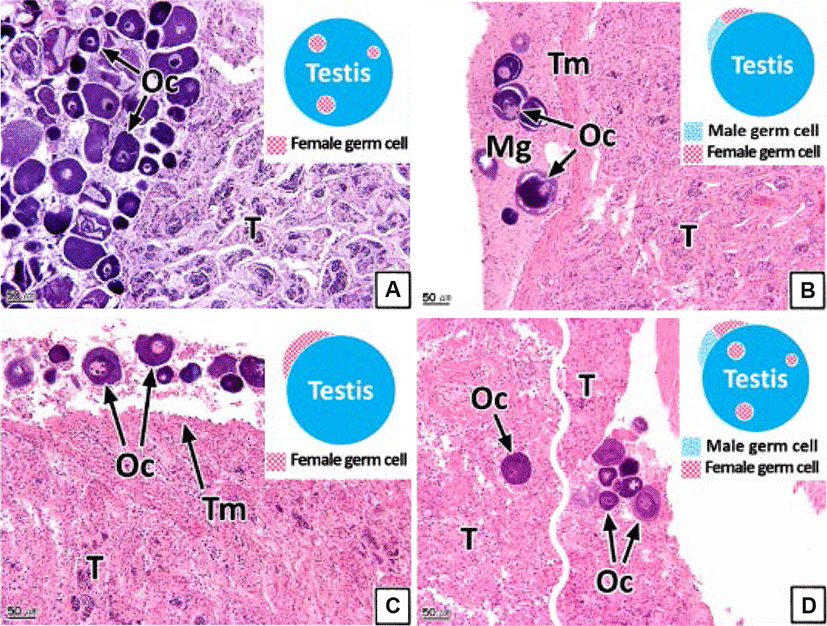
Intersex is one of the pathological sexual abnormalities that manifest due to environmental factors, such as the presence of endocrine-disrupting chemicals or heavy metals (Bortone & Davis, 1994). Studies of such abnormalities in teleosts have been undertaken in various species including Gambusia affinis and largemouth bass (Drysdale & Bortone, 1989; Blazer, 2002; Barnhoorn et al., 2010; Lee et al., 2010; Bahamonde et al., 2013; Mankidy et al., 2013).
Two primary causes of intersexuality and structural abnormalities in the gonads of rock bream can be confirmed from the results of the present study. The first cause is the presence of chemical factors such as EDC’s in their habitat. The second cause is the chemical components contained in mixed feeds, which are artificially supplied during the early life stages of artificial production of young rock bream. However, more detailed studies are required to ascertain the reasons for intersexuality and gonad structural abnormalities.