INTRODUCTION
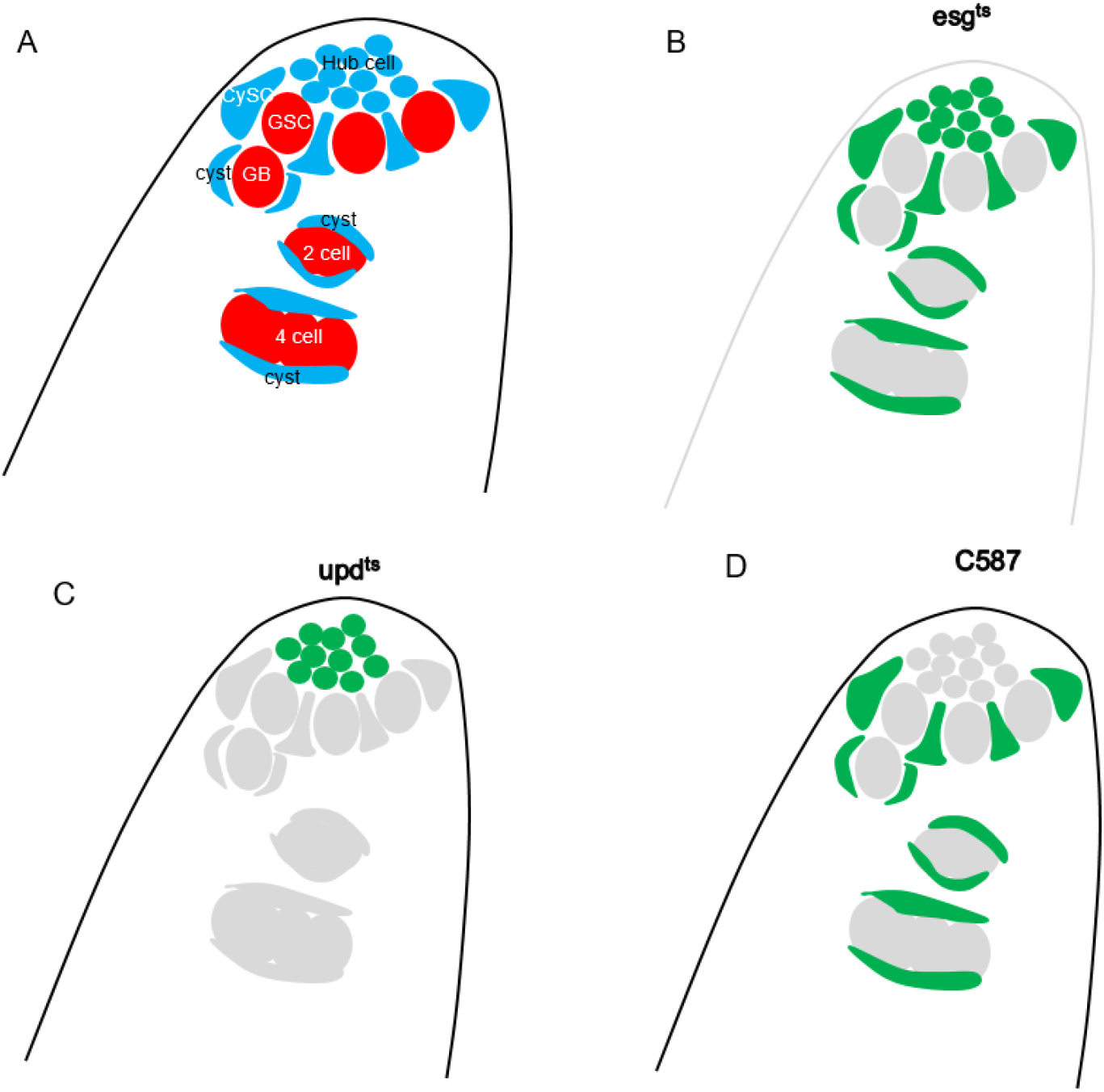
The soma/germline distinction is essential to the survival of all animal species; in the absence of germline cells, sperm and eggs cannot be produced, leading to termination of the species. In Drosophila, primordial germ cells (PGCs) are specified during early embryogenesis in the germ-plasm at the posterior end of the embryo (Lehmann, 2016; Dehghani & Lasko, 2017). Initially, they migrate anteriorly and become encapsulated with somatic cells to form embryonic gonads (Boyle & DiNardo, 1995; Rongo et al., 1997; Okegbe & DiNardo, 2011; Anllo et al., 2019; Anllo & DiNardo, 2022). Later, the PGCs differentiate into germline stem cells (GSCs) while the gonadal somatic cells differentiate into hub and cyst stem cells (CySCs), forming the adult testis (DiNardo et al., 2011; Losick et al., 2011). At the tip of the adult testis is the hub, comprised of ~10 cells, to which are attached intermingled CySCs and GSCs (Yamashita et al., 2003, 2005; Davies & Fuller, 2008). The hub secretes signaling molecules including Unpaired (Upd), Bone morphogenetic protein, and Hedgehog, which stimulate CySCs and GSCs for hub attachment, asymmetric cell division, and stemness maintenance (Kiger et al., 2001; Tulina & Matunis, 2001; Leatherman & Dinardo, 2008, 2010; Amoyel et al., 2013). The CySCs produce cyst cells that encapsulate GSC-derived differentiating germ cells (de Cuevas & Matunis, 2011; Spradling et al., 2011).
Germline-specific genes are exclusively expressed in the PGCs of embryos and the germline cells of adult gonads (Lehmann & Nüsslein-Volhard, 1991; Rongo et al., 1997; Slaidina & Lehmann, 2014; Trcek & Lehmann, 2019). The mechanisms that restrict expression of germline genes are yet poorly understood. The evolutionarily conserved DEAD-box RNA helicase Vasa (also known as DDX4) is one such germline-specific gene, being expressed in germline cells from the early embryo to the late adult gonad (Lasko & Ashburner, 1990; Rongo et al., 1997; Van Doren et al., 1998; Wang et al., 2015; Jeske et al., 2017). In Drosophila, it is involved in germline specification in embryos and in GSC maintenance and differentiation in adults (Lasko, 2013; Dehghani & Lasko, 2017; Durdevic & Ephrussi, 2019; Adashev et al., 2024). Mechanistically, Vasa binds hundreds of mRNAs, is required for the enrichment of several hundred mRNAs at the posterior pole in embryos, and is involved in the translational regulation of selected mRNAs (Lasko, 2013; Kotov et al., 2024). In this communication, we report expression of a miR932 sponge to result in ectopic vasa expression in hub cells, revealing a microRNA-based mechanism regulating vasa expression in the adult testis.
MATERIALS AND METHODS
Animals were maintained on a standard cornmeal diet (68 g dry yeast, 90 g sugar, 43 g cornmeal, 9 g agar, 4.5 mL propionic acid, 1 g methyl-4-hydroxybenaoate per 1-liter water) at 25°C and 40% relative humidity under 12-hour light/dark cycle conditions. All flies harboring esgts, updts>UAS-miR.sponge were raised at 22°C to restrict Gal4 unless otherwise noted. Flies were shifted to 29°C for three days to inhibit Gal80ts and activate Gal4.
The following lines were generous gifts from colleagues in the fly community: esgts driver refers to esg-Gal4, UAS-GFP/Cyo; tub-Gal80ts (Micchelli & Perrimon, 2006), and updts driver refers to upd-Gal4; tubP-Gal80ts (Albert et al., 2018). The following lines were obtained from the Bloomington Drosophila Stock Center: C587-Gal4 (BL67747), UAS-mCherry.scramble.sponge (BL61501), UAS-mCherry.miR-932.sponge (BL61439), and UAS-mCherry.miR-let7.sponge (BL61635).
Testes were dissected in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde in 1XPBS for 30 minutes at room temperature. Fixed samples were washed twice with 0.3% triton X-100 in 1XPBS (1XPBST) for 15 minutes at room temperature, then blocked with 5% normal goat serum in 1XPBST (blocking solution). Primary antibodies were diluted in blocking solution and incubated overnight at 4°C. Testes were washed twice with 1XPBST for 15 minutes each time and incubated with secondary antibodies for two hours at room temperature. After the incubation, the testes were again washed twice with 1XPBST for 15 minutes, then mounted in Fluoromount-G® (Southern Biotech, Birmingham, AL, USA) on a glass slide. Primary antibodies were rabbit anti-mCherry (PA5-34974, 1:200 Invitrogen, Waltham, MA, USA), rabbit anti-eGFP (CAB4211, 1:500 Invitrogen), rat anti-Vasa (760351, 1:400 Developmental Studies Hybridoma Bank, DSHB, Iowa, IA, USA), and mouse anti-FasIII (7G10, 1:30 DSHB). Secondary antibodies were as follows: Alexa Fluor 488-conjugated goat anti-rabbit (A11008, Invitrogen, diluted 1:800), Alexa Fluor 488-conjugated goat anti-mouse (A11001, Invitrogen, 1:800), Alexa Fluor 555-conjugated donkey anti-mouse (A31570, Invitrogen, 1:800), and Alexa Fluor 555-conjugated goat anti-rabbit (A21429, Invitrogen, 1:800). Images were taken with a Leica Application Suite X confocal microscope system and image analysis was performed using the Leica LAS X software.
RESULTS AND DISCUSSION
Vasa is expressed in germline cells, including GSCs, the goniablast (GB), and GB-derived germline cells of the adult testis; conversely, it is not expressed in somatic cells of the adult testis, including hub cells, CySCs, and the cyst (Fig. 1A). To identify microRNAs functioning in the Drosophila adult testis, we employed the Gal4/UAS binary expression system (Brand & Perrimon, 1993), which enables expression of UAS-transgenes under a tissue-specific Gal4 driver. This study used the temperature-sensitive somatic Gal4 driver termed esgts, which harbors esg-Gal4 (somatic-Gal4) and tub-Gal80ts (tubulin promoter linked to a temperature-sensitive form of the Gal4 inhibitor Gal80) (Micchelli & Perrimon, 2006). After a temperature shift from 25°C to 29°C, which renders Gal80 nonfunctional, esgts can drive expression of UAS-transgenes in somatic cells (Fig. 1B). To knock down microRNAs, we employed UAS-microRNA sponges (Fulga et al., 2015) which consist of the mRNA for mCherry with twenty concatenated copies of a sequence complementary to a microRNA inserted into its 3’UTR. These antisense sequences can sequester microRNAs, allowing expression of the transcripts the microRNAs would otherwise repress.
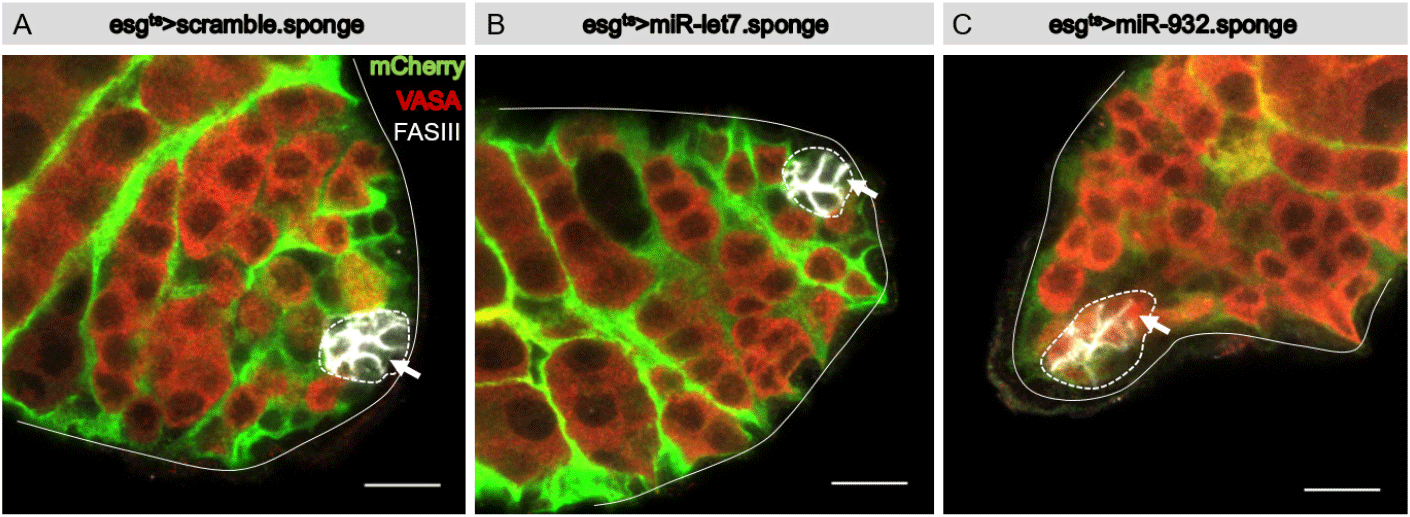
Flies were reared at 22°C for three days after eclosion, then shifted to 29°C for three days. Testes were dissected out and immunostained with a hub-specific antibody (FasIII), germline-specific antibody (Vasa), and mCherry-specific antibody to label cells in which the Gal4 driver was active. Vasa was detected in germline cells of control and experimental testis (Fig. 2A–C). Vasa was not detected in hub cells of control testis (esgts>UAS-mCherry.scramble.sponge and esgts>UAS-mCherry.miR-let7.sponge) (Fig. 2A and B), but was found to be ectopically expressed in hub cells of the esgts>UAS-mCherry.miR-932.sponge testis (Fig. 2C), indicating that knockdown of miR-932 by the miR-932 sponge induced ectopic expression of vasa in hub cells. This suggests that miR-932 acts to silence vasa expression in hub cells of the wild-type testis.
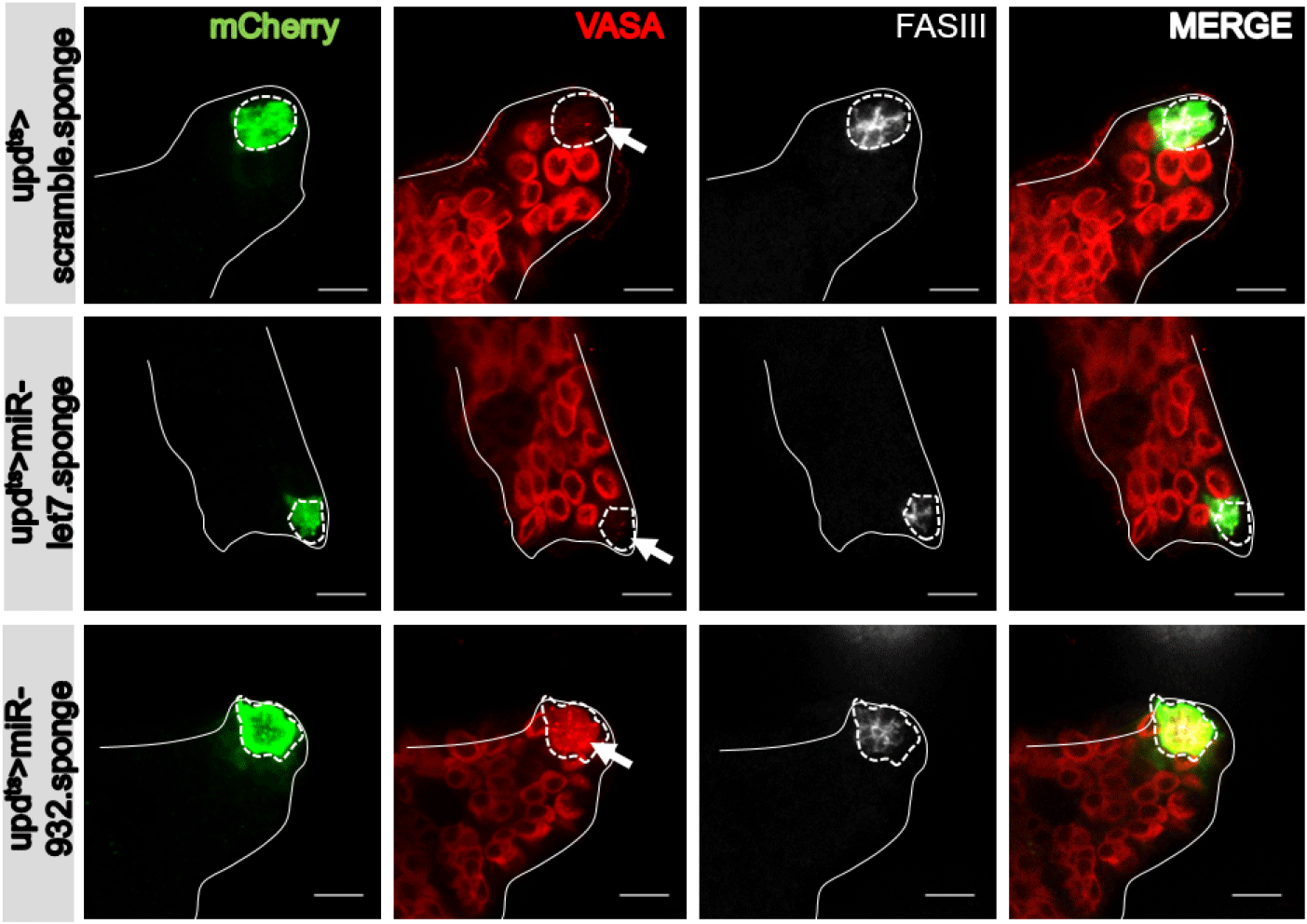
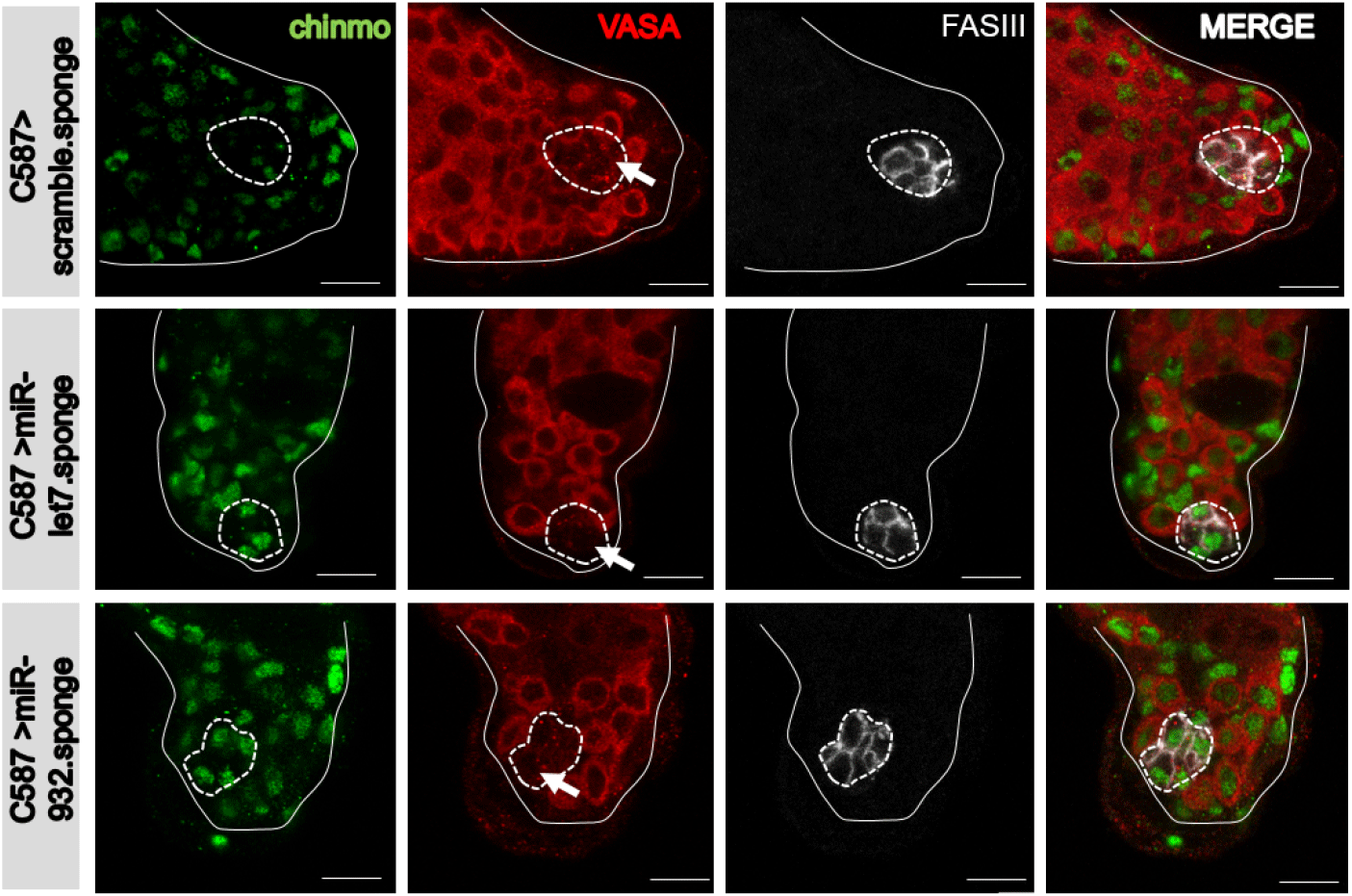
Esgts drives expression of UAS-transgenes in somatic cells, inclusive of hub cells, CySCs, and cyst cells (Fig. 1B). To examine whether the effect of miR932 sponge expression on vasa expression in hub cells is intrinsic (cell-autonomous) or extrinsic (non-cell-autonomous) in mechanism, we employed the hub-specific updts driver (Albert et al., 2018) that harbors upd-Gal4 (a hub-specific Gal4) and tub-Gal80ts (Fig. 1C). At three days post-shifting to 29°C, ectopic vasa expression was also observed in the hub cells of updts>UAS-mCherry.miR-932 sponge flies (Fig. 3), supporting a cell-autonomous (or intrinsic) effect of miR-932 sponges on expression of vasa in hub cells. Ectopic vasa expression was not observed in control testis (updts>UAS-mCherry.scramble.sponge and updts>UAS-mCherry.miR-let7.sponge) (Fig. 3). Expression of the miR-932 sponge under the C587-Gal4 driver (specific to CySCs and cyst cells) (Fig. 1D) (Le Bras & Van Doren, 2006) did not produce ectopic expression of vasa in CySCs or cyst cells (Fig. 4). Thus, the miR-932 sponge, which is likely to knock down miR-932, acts intrinsically to facilitate ectopic expression of vasa in hub cells, but not in CySCs or cyst cells.
CONCLUSION
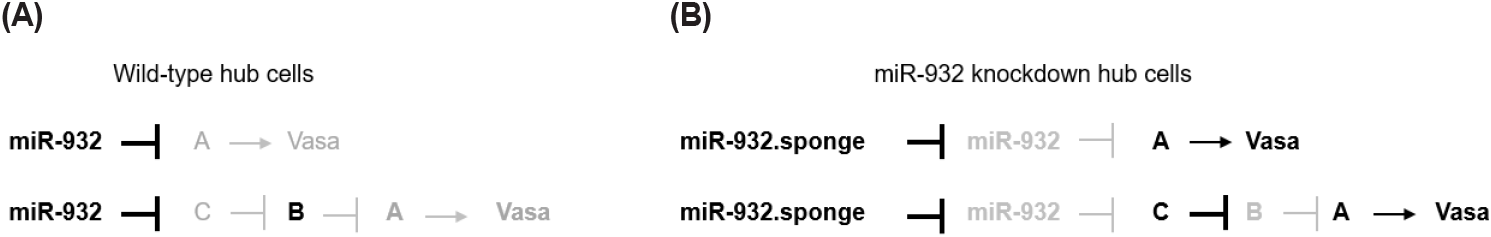
We demonstrated that the specific expression of the miR-932 sponge in hub cells resulted in the ectopic expression of vasa. These findings suggest that vasa is inhibited by miR-932 in hub cells from wild-type testis. An examination of the vasa transcript did not identify any miR-932 complementary sequences, which excludes the possibility of its direct inhibition by miR-932. Thus, miR-932 might target other regulators that control vasa expression in hub cells (Fig. 5). MiRNA target prediction software (TargetScanfly 7.2) identified 163 transcripts with miR-932 binding sites, including histone deacetylase 4 (HDAC4) and 65 transcripts of unknown function. The knockdown of these targets in hub cells using UAS-RNAi lines, available in Drosophila stock centers from the USA, Japan, and Europe, could lead to the identification of miR-932 targets. Future research identifying hub-cell-specific miR-932 targets with ectopic vasa expression is required to elucidate the mechanisms that prevent vasa expression in hub cells of the adult testis. It is worth noting that the expression of the miR-932 sponge in other somatic cells (CySCs and cyst cells) did not result in ectopic vasa expression. Thus, mechanisms other than miR-932 may exist for inhibiting vasa expression in CySCs and cyst cells.