INTRODUCTION
It is known that there are about 212 genuses and 1,875 species of the fishes of Gobiidae are known to exists around the world, and in Korea about 27 genuses and 74 species are reported (NIBR, 2011). Trident goby, Tridentiger obscurus belongs to Perciformes order, Gobiidae family, and Tridentiger genus, and lives fresh water and brackish water zone of estern and western·southern coast of Korea, Han river, Geum river, Nakdong river, and Jeju Island (Kim et al., 2005). As for morphological characteristics, it has blunt mouth and tongue of short width, and jaws of the same length on both sides. Small teeth are arranged in two rows, the teeth of external rows are all composed of triangular peaks except for 2–3 teeth in the back.
As for the researches on Tridentiger obscurus, there are researches on taxonomic research (Kim & Choi, 1989), taxonomic consideration (Kim & Yang, 1995), sucker structure (Kim et al., 2002), early life history (Hwang et al., 2006) and reproductive cycle (Jin et al., 2006). In Korea, there were researches on early life history of Gobiidae fishes such as Chasmichthys dolichognathus (Kim, 1975), T. trigonocephalus (Kim & Han, 1990), Mugilogobius abei (Kim & Han, 1991), Favonigobius gymnauchen (Jin et al., 2003), L. grandis (Yoon et al., 2008), but researches on skeletons of Gobiidae are conducted on Chaenogobius laevis (Kim & Han, 1989), Periophthalmus modestus (Lee, 1990), and Luciogobius guttatus (Kim et al., 1992) with a few researches on skeletons.
When it comes to brood stock culture of fishes, researches on skeleton development of Larvae and juvenile are essential for identifying and removing skeleton abnormalities in the early stage of culture, and they can be an important data not only for development of species, but also of skeletal characteristics of adult fishes. Therefore, concrete and systematic researches are needed (Koumoundouros et al., 1997a,b). Therefore, in this study, we would like to observe the developmental process of the larvae and juvenile skeleton of the T. obscurus and use it as the basic data of the taxonomic study.
MATERIALS AND METHODS
For securing brood stock, was captured with a skimming net in the river estuary of Jeollanam-do, Yeosu-si Ocheon-dong. The total length of females is 6.3–7.0 (mean 6.64±0.27, n=6) cm, that of males is 7.2–8.0 (mean 7.63± 0.29, n=5) cm. Brood stock was transported to a research room after being packed in a vinyl, and cultured in a glass square tank (35×50×30 cm) of a recirculation culture system.
Brood stock being cultured spawn eggs in a pipe made of PVC within tank, the temperature of the eggs was maintained at 18.4℃-23.2℃ (mean 21.2℃), and the water was changed by more than 50% twice a day. For larvae and juvenile culture, salt concentration was maintained at 30.3–33.0 psu (mean 32.4 psu) and the temperature was the same as the management condition of the fertilized eggs. As for the feed, larvae of rotifer, Brachionus rotundiformis and Artemia sp. nauplius in consecutive order.
For observation of skeleton development of larvae, during the period from incubation to juvenile, 10 fishes were randomly selected and fixed on neutral formalin of 5%. The fixed samples were double strained according to the method of Kawamura & Hosoya (1991). All-purpose projector (Nikon JP V-12B, Japan) and stereoscopic microscope (Nikon NM-40, Japan) were used to observe skull, jawbone, visceral skeleton, iliotibial bone and tailbone, and the size was measured to 0.01 mm. The name of each part was followed by Kim & Han (1989).
RESULTS
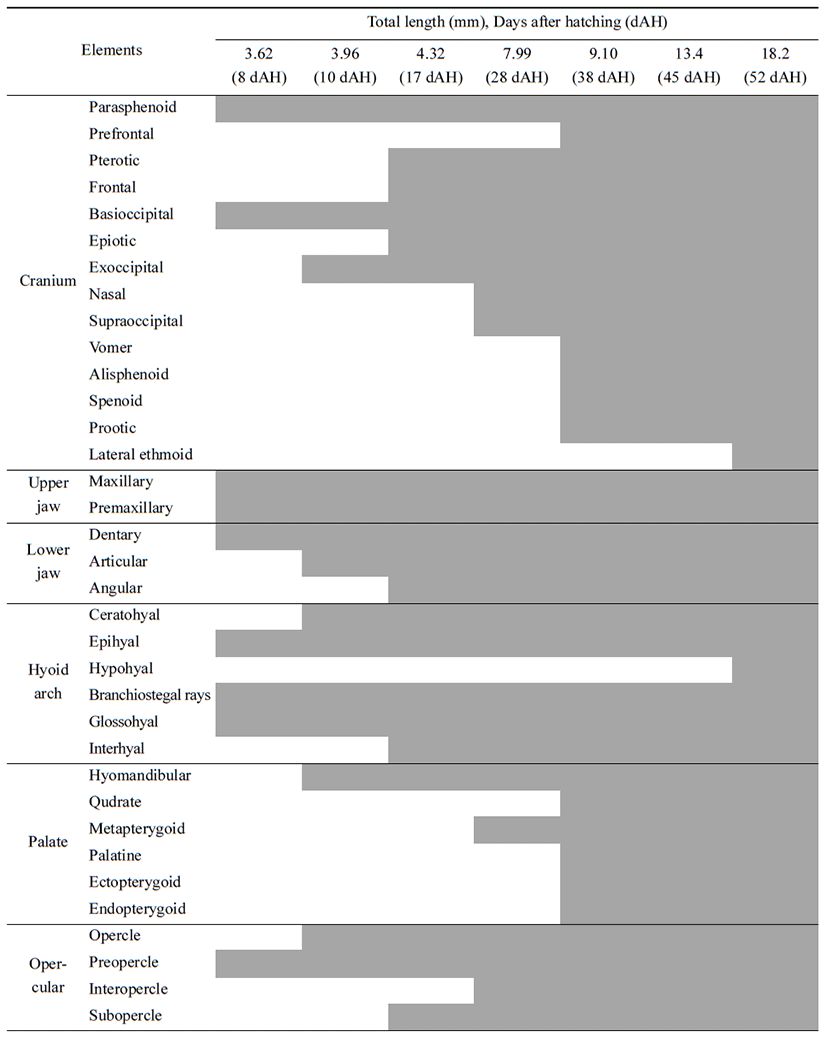
Head skeleton of fishes is composed of cranium and visceral skeleton, pectoral girdle bone is jointed with base occipital bone of skull. In the operculum part, preopercle began to ossify (Tables 1-2), 8 days after hatching when ossification of the first skeleton was proceeded, preflextion larvae is 2.83–4.07 mm (mean 3.62 mm) ossification was conducted in parasphenoid of line shape on cranium. On the back, basioccipital began to ossify. Premaxillary and maxillary of upper jaw which function related to feeding ossified, and dentary of lower jaw ossified. In the part of tongue, epihyal ossified, and in the operculum, preopercle bagan to ossify, and in the pectoral girdle. clavicle bagan to ossify (Fig. 1A).
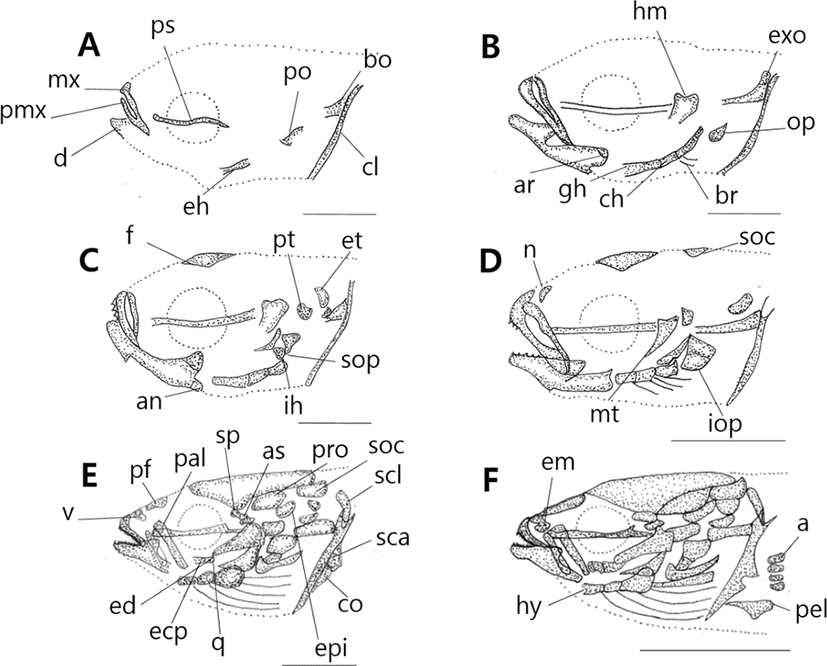
10 days after hatching, flextion larvae of the mid term is 3.83–4.43 mm of total length (mean 3.96 mm), and in the cranium exoccipital ossified, and hyomandibular ossified in palatal part. Opercle ossified in the operculum part, articular ossified in the lower jaw, and branchiostegal rays, ceratohyal and glossohyal began to ossified (Fig. 1B).
17 days after hatching, postflextion larvae of the latter period is 3.63–4.93 mm of total length (mean 4.32 mm), in the head bone, pterotic, epiotic, and frontal ossified, and interhyal in the tongue part ossified, subopercle ossified in the operculum, and angular ossified in the lower jaw (Fig. 1C).
28 days after hatching, postflextion larvae of hatch is 7.18–8.73 mm of total length (mean 7.99 mm). Nasal and supraoccipital ossified in the cranium, metapterygoid ossified in palate part, and interopercle ossified in the operculum part (Fig. 1D).
38 days after hatching, postflextion larvae of the latter period is 8.03–10.0 mm of total length (mean 9.10 mm). Spenoid, vomer, prootic, prefrontal and alisphenoid ossified in the cranium. Palatine, ectopterygoid, endopterygoid, and quadrate ossified, supracleithrum, scapula, and coracoid ossified in pectoral girdle (Fig. 1E).
52 days after hatching, juvenile is 16.6–19.2 mm of total length (mean 18.2 mm). As lateral ethmoid ossifies in the cranium, hypohyal in the tongue part, actinost in the pectoral girdle, and pelvic girdle in the lower part, completing ossification of all skeletons.
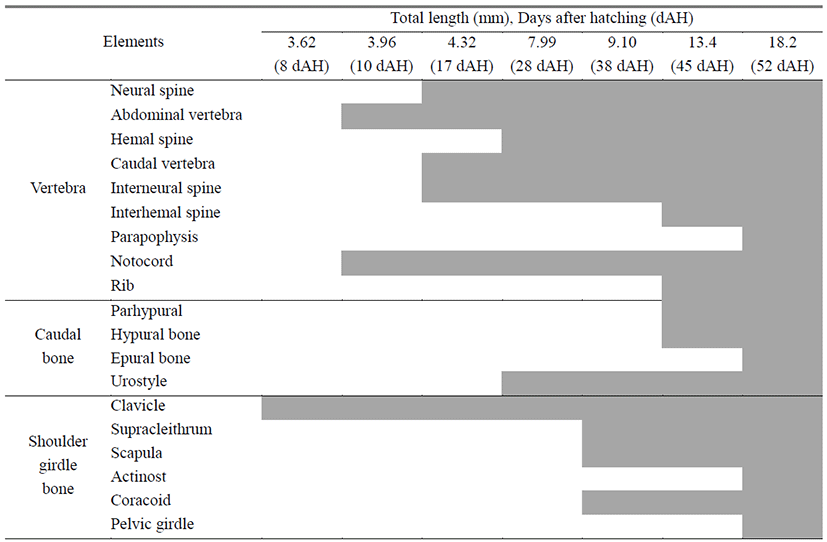
Vertebrae is the caudal skeleton of a body and protecets spinal cords and blood vessels. Ossificaion direction of vertebrae proceeds from head to tail.
10 days after hatching, flextion larvae of the mid term is 3.83–4.43 mm (mean 3.96 mm) and is composed of notochord. Vertebrae’s ossification begins with 8 abdominal vertebrae on the front (Fig. 2A). 17 days after hatching, larva of the latter period is 3.63–4.93 mm (mean 4.32 mm), the number of abdominal vertebrae increases to 10, and 4 caudal vertebrae on the back begin to ossify (Fig. 2B).
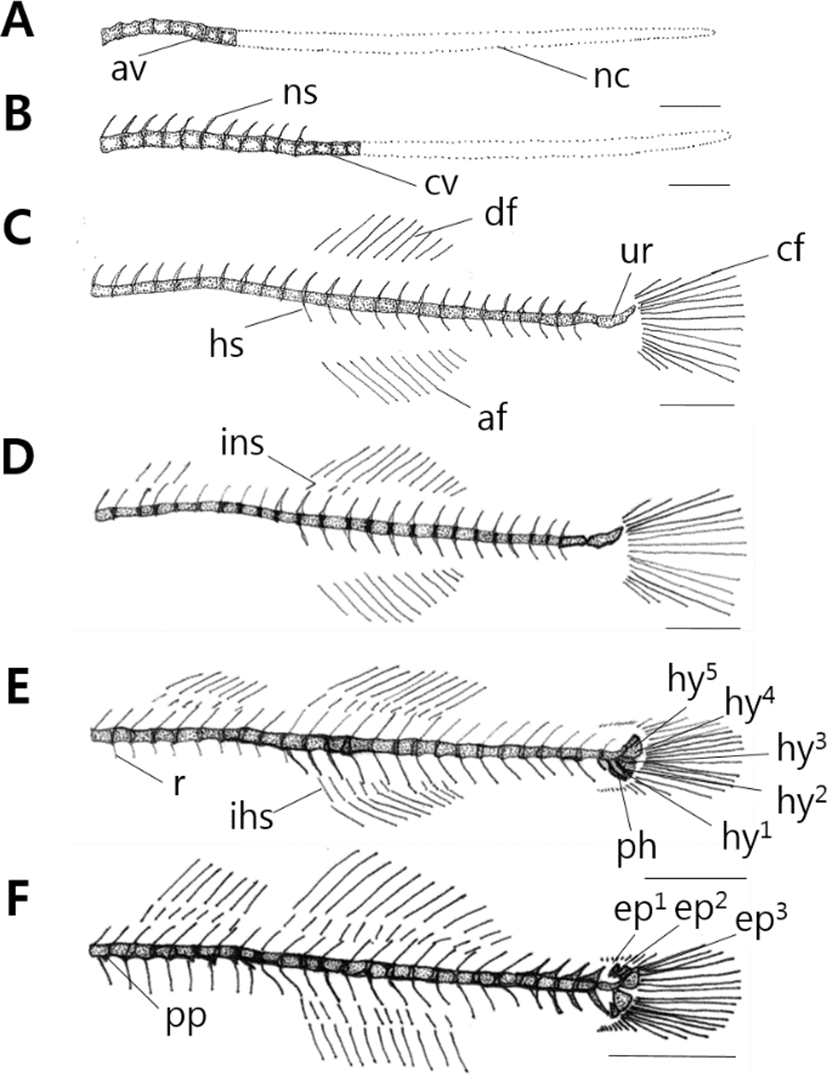
28 days after hatching, postflextion larvae of the latter term is 7.18–8.73 mm (mean 7.99 mm), stems of back, rear, tail fin form, and the number of caudal vertebrae increases to 15. During this period, the number of abdominal vertebrae is 10–11, the number of caudal vertebrae is 15–16, and a total of 25–27. The number of neural spine increases to 24, henal spine to 14. As urostyle of tailbone curves by 45°, ossification begins (Fig. 2C).
38 days after hatching, postflextion larvae of the latter term is 8.03–10.0 mm (mean 9.10 mm) 3 interneural spines which support the second back fin ossify (Fig. 2D).
45 days after hatching, postflextion larvae of the latter term is 11.6–14.3 mm (mean 13.4 mm), and rib ossifies in the upper part of abdominal vertebrae. The number of the first back fin interneural spine increases to 6, the second back fin interneural spine to 10, and the interhemal spine supporting the back fin to 10. In the tail bone part, 3 hypural bones and 1 parhypural bone ossify (Fig. 2E).
52 days after hatching, juvenile of the latter term is 16.6–19.2 mm (mean 18.2 mm). In the lower part of abdominal vertebrae, parapophysis ossifies, in the upper part of urostyle, 3 epural bones ossify. As the number of hypural bone increases to 5, the ossification of vertebrae and tail bones becomes completed.
DISCUSSION
Tridentiger obscurus showed no skeletal development right after hatch. From the time when the total length is 3.62 mm after hatch, maxillary, premaxillary, and preopercle which play the role of feeding and respiraton first ossified. In the skull, parasphenoid and basioccipital begin to ossify. This skeletal development was similarly seen in Luciogobius guttatus (Kim et al., 1992) and Chaenogobius laevis (Kim & Han, 1989). As the mouth and swimming ability develop, clavicle of pectoral girdle develops. On the 8 days after hatching, Tridentiger obscurus shows ossification of clavicle when it is 3.62 mm of the total length, consecutively on the 38 days after hatching, supracleithrum, scapula, and coracoid ossify and it is 9.10 mm long.
Chaenogobius laevis of the same Gobiidae family shows the first ossification of clavicle on the 13 days after hatching, on the 17 days after hatching, supracleithrum ossifies, and on the 43 days after hatching actinost ossifies, completing ossification of pectoral girdle (Kim & Han, 1989). Tridentiger obscurus completed ossification of pectoral girdle among comparative species. Clavicle first ossifies, and actinost lastly ossifies in the same way.
Vertebrae is the central skeleton of a body. Ossificaion direction of vertebrae proceeds from head to tail consecutively. As abdominal vertebrae ossifies, interneural spine begins to ossify in the upper part. As tail bones ossify, hemal spine ossifies. Chaenogobius laevis of the same Gobiidae family showed similar ossification to Tridentiger obscurus. However, vertebrae ossified before the completion of ossification of urostyle, showing different ossification from Tridentiger obscurus and similar to ossisificatio process of Luciogobius guttatus (Kim et al., 1992) and Chaenogobius laevis (Kim & Han, 1989).
Pterygiophore which supports fins ossified from the front to the back. Tridentiger obscurus and Chaenogobius laevis (Kim & Han, 1989), Luciogobius guttatus (Kim et al., 1992) developed the second back fin at the time when interneural spine ossified, showing the same development.
Research results suggest that development is the same as the feeding and respiration functions first develop among similar species. Tridentiger obscurus completed ossification the fastest of clavicle among similar species. Vertebrae ossified the same way as urostyle, but Chaenogobius laevis (Kim & Han, 1989) and Luciogobius guttatus (Kim et al., 1992) showed differences in development as vertebrae ossified before the ossification of urostyle.
Researches on skeletal development can be an important data for analyzing official phenomenon and malformation development among carnivorous fish. Gobiidae fish are not main species for culture, but the characteristics shown in the skeletal development process can be used as taxology database. These researches are thought to serve as an important research for classifying similar species.