INTRODUCTION
The endoplasmic reticulum (ER), a cellular organelle, is well known as a modulator of protein folding and calcium homeostasis. As a result of various ER functions, ER stress is induced by the accumulation of unfolded proteins in the ER lumen (Raab et al., 2009). To inhibit the ER stress, UPR (Unfolded protein response) signaling is activated for cell survival. Under ER stress, three UPR transducers, protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1), dissociate from their binding partner, Glucose-Regulated Protein, 78-kDa (BIP/GRP78), which then binds to the unfolded proteins (Wu & Kaufman, 2006). First, to prevent new protein synthesis in the stressed ER, the activated PERK phosphorylates eukaryotic initiation factor 2α (eIF2α) to inhibit translation initiation. However, phosphorylated eIF2α subsequently induces the expression of activating transcription factor 4 (ATF4). Second, P90ATF6 is cleaved by site-1 protease (S1P) and site-2 protease (S2P) in the Golgi compartment and converted into activated P50ATF6. Third, phosphorylation of IRE1 induces the splicing of x-box binding protein 1 (XBP1), which converts it into a more potent transcription factor that regulates the transcription of ER chaperones. IRE1 signaling inhibits protein synthesis and induces ER-associated degradation (ERAD) by the ubiquitin-dependent proteasomal pathway (Teske et al., 2011a). Finally, repetitive and persistent ER stress induces the activation of CCAAT-enhancer-binding protein homologous protein (CHOP), which regulates the transcription of apoptotic factors during ER stress (Namba & Kodama, 2015).
BIP/GRP78 is a major chaperone protein for protein quality control in the ER (Wang et al., 2009). In particular, BIP/GRP78 is a critical regulator that controls the activation of ER-transmembrane signaling mechanisms, such as ER-stress-induced activation of three pathways of UPR. Further, it plays an important role in cell proliferation, differentiation, and protection of the inner cell mass of blastocysts from apoptosis during embryonic development (Luo et al., 2006). BIP/GRP78-mediated activation of three UPR signal pathways is also involved in the reproduction system, such as apoptosis regulation in granulosa cells (Farinola et al., 2007), and cell cycle arrest in lung cancer cells (Zhu et al., 2015). In addition, many studies demonstrated that the inhibition of ER stress is involved in meiotic maturation, parthenogenetic embryonic development in vitro(Zhang et al., 2012b), ovarian granulosa cell apoptosis (Yang et al., 2013), embryonic development of mice (Zhang et al., 2012a), and granulosa cell apoptosis during follicular atresia in goat (Lin et al., 2012). However, the role of BIP/GRP78-related ER stress or activation of UPR signaling pathways in in vitro maturation (IVM) of porcine oocytes has not been reported.
Therefore, we investigated the effect of BIP/GRP78-regulated ER stress on porcine oocyte maturation. The objective of the current study was to determine whether BIP/GRP78-regulated ER stress affects meiotic maturation and UPR signaling gene expression in the porcine oocytes.
MATERIALS AND METHODS
Unless indicated otherwise, all chemicals in this study were purchased from Sigma Aldrich Korea (St. Louis, MO, USA).
We performed IVM as described by Kim et al. (2016), but with slight modification. Pig ovaries were collected from a local slaughterhouse and transported to our laboratory at 35℃ in 0.9% saline supplement with 75 μg/mL potassium penicillin G. Immature cumulus-oocyte-complexes (COCs) between 3 and 6 mm in diameter were aspirated into a 10 mL syringe from ovary follicles by using an 18-gauge needle. Subsequently, undamaged aspirated COCs with similar quality cytoplasm and with oocytes surrounded by cumulus cells were selected by using mouth pipettes. The selected COCs were washed three times in TL-HEPES medium, and approximately 50 COCs were matured in 500 μL of IVM medium at 38.5℃ and under 5% CO2 in air. The IVM medium was NCSU-23 medium with 0.57 mM cysteine, 10% porcine follicular fluid, 10 ng/mL epidermal growth factor (EGF), 10 ng/mL β-mercaptoethanol, 10 IU/ mL pregnant mare serum gonadotropin (PMSG), and 10 IU/mL human chorionic gonadotropin (hCG). After culturing for 22 h, COCs were washed three times and then cultured further in IVM medium without PMSG and hCG for 22 h. And then, we collected the matured porcine COCs at the 44 h after IVM.
After 44 h of IVM, meiotic maturation was distinguished by nuclear stages. Oocytes were denuded by pipetting in TL-HEPES medium containing 0.1% hyaluronidase, washed in PVA-PBS and mounted on microscope slides. The samples were fixed for 3 days in acetic acid/ethanol (1: 3, v/v), stained with 1% acetic orcein (v/v) for 5 min. Meiotic stage of the samples was evaluated under a microscope (Leica, Solms, Germany).
Total RNA were extracted from porcine COCs using TRIzol reagent (Invitrogen, CA) according to the manufacturer’s instructions. Extracted RNA was quantified by a NanoDrop spectrophotometer (ACTgene, Piscataway, NJ). Each cDNA was synthesized from the aliquots (1 μg/μL) of total RNA with AccuPower® RT-PCR Premix (Bioneer, Korea). PCR was carried out using AccuPower® PCR Premix (Bioneer) containing specific primers: BIP/GRP78; GGTGGGCAAACAAAGACATT (sense) and CGCTGGT CAAAGTCTTCTCC (antisense). Specific primer sequences were designed using the NCBI database.
Following the 44 h IVM period, lysates of 30 COCs were prepared in protein lysis buffer PRO-PREP (iNtRON Biotechnology, Seoul, Korea) with centrifugation at 13,000 rpm for 10 min at 4℃. Sample lysates were separated on 12% polyacrylamide gel by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes (Pall Corporation, NY, USA). The membranes were blocked by incubation with 5% skim milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 overnight at 4℃. Membranes were then incubated with the appropriate primary antibody: antibody anti-GRP78 (1 : 2,000; Santa Cruz Biotechnology, CA, USA), anti-ATF4 (1 : 5,000; Santa Cruz), anti-P90 ATF6 (1 : 4,000; Novus Biologicals, USA), anti-β-ACTIN (1 : 3,000; Santa Cruz), anti-p-eIF2α (1: 2,000; cell signaling) and anti-eIF2α (1: 2,000; cell signaling) antibodies. Following incubation, membranes were washed and incubated with secondary antibody HRE-conjugated anti-mouse/ rabbit IgG (Thermo Fisher Scientific, Rockford, IL, USA) for 3 h at room temperature. Antibody binding was detected by using a chemiluminescent system (Bight ECL Kit, Advansta, Menlo Park, CA, USA) according to the manufacturer’s instructions. Band intensities were quantified by using Image J software (National Institutes of Health, MD).
Three predesigned potential BIP/GRP78 siRNAs were chemically synthesized by GenePharma (Shanghai, China). Maturated COCs were transfected with BIP/GRP78 siRNA (100 nM) and negative control siRNA (25 nM) using lipofectamine 3000 according to the manufacturer’s instructions. Sequences of porcine BIP/GRP78 and negative control siRNAs were as following : #909: CCU UCU CAC CAU UGA UAA UTT (sense), AUU AUC AAU GGU GAG AAG GTT (antisense); #693: GGG AAA GAA GGU UAC UCA UTT (sense), AUG AGU AAC CUU CUU UCC CTT (antisense); #1570: GCC UCU GAU AAU CAG CCA ATT (sense), UUG GCU GAU UAU CAG AGG CTT (antisense); negative control siRNA: UUC UCC GAA CGU GUC ACG UTT (sense), ACG UGA CAC GUU CG AGA ATT (antisense).
After IVM 44 h after siRNA treatment, COCs in M II (44 h) were washed with 0.3% PVA-PBS and fixed in 4% (v/v) paraformaldehyde and 2.5% (v/v) glutaraldehyde solution for 1 h at room temperature. Next, COCs were transferred to a permeabilization solution (0.2% Triton X-100) at room temperature for 1 h and then incubated in 0.3 % PVA-PBS overnight at 4℃. After blocking overnight at 4℃ in 0.1% PVA-PBS containing 1% BSA, the COCs were incubated with anti-BIP/GRP78 (sc-1050; Santa cruz) diluted 1 : 500 at 4℃ overnight. After incubation, the COCs reacted with the secondary antibody, FITC-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology), diluted 1 : 1000 in 0.3% PVA-PBS for 2 h at room temperature. DAPI reagent (2 mg/mL) was used to stain the nuclei. Immunoreactivity was observed under a Zeiss LSM 700 confocal microscope (Carl Zeiss, Oberkochen, Germany).
All percentage data and data sets were subjected to arsine transformation and expressed as the mean ± standard deviation (SD). And all values of Western blot experiments were presented as the mean ± standard error of the mean (SEM). The results were analyzed using a one-way ANOVA followed by Bonferroni’s Multiple Comparison Test and using t-tests. All data were performed using the GraphPad Prism 5.0 software package (San Diego, CA, USA). Differences were considered significant at *p<0.05, **p<0.01, and ***p<0.001.
RESULTS
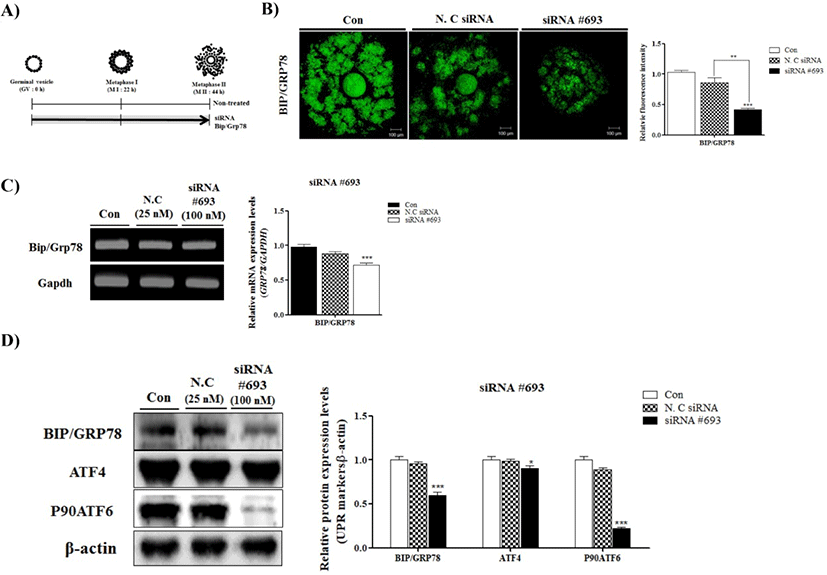
To observe the effects of BIP/GRP78 on oocyte maturation, we used specific siRNA (#693, #1570 and #909) of BIP/GRP78 for pig (data not shown). After siRNA #693, #1570 and #909 treatment, we chose the most appropriate depending on transfection efficiency. Over all, we determined the siRNA #693 of BIP/GRP78 for use in subsequent experiments.
Changes in BIP/GRP78 fluorescence expression in negative control of siRNA and siRNA #693 transfected COCs were investigated by immunofluorescence staining. As shown in Fig. 1B BIP/GRP78 fluorescence intensity was lower (p<0.001) in siRNA #693 of BIP/GRP78 transfected COCs than other treated groups (Fig. 1B). In addition, to investigate the mRNA and protein levels of BIP/GRP78, we performed RT-PCR analysis and Western blotting, respectively. As a result, mRNA and protein levels of BIP/ GRP78 significantly decreased (p<0.001) in the BIP/GRP78 siRNA #693 treated COCs compared with control and negative siRNA treated groups (Fig. 1C and 1D). Moreover, ATF4 and P90ATF6 protein levels were reduced the in siRNA #693 transfected COCs. These results indicated that the reduction of BIP/GRP78 expression by siRNA #693 reduced the UPR marker proteins expression of COCs at 44 h of IVM in porcine oocytes.
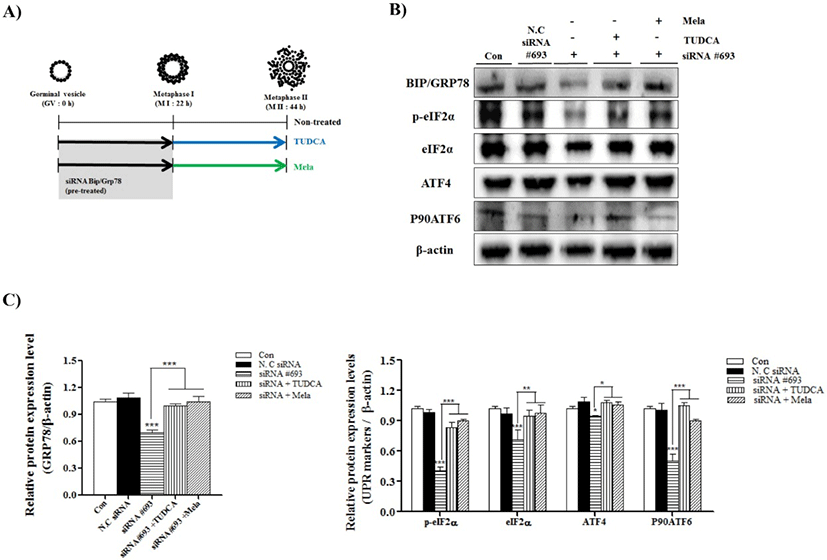
To confirm the induced ER-stress by reduction of BIP/ GRP78 gene expression on porcine oocyte maturation, we used the IVM medium supplemented with ER-stress inhibitor, TUDCA (200 μM) and melatonin (0.1 μM) (Fig. 2A: mimetic diagram of experiment methods). First, we investigated the meiotic maturation by acetic-orcein staining (Table 1). The meiotic maturation was reduced (p<0.05) in COCs of siRNA #693 treated groups compared with control and other treated groups (Table 1). As expected, meiotic maturation significantly recovered (p<0.001; siRNA #693: 32.5 ± 10.1% vs siRNA #693+TUDCA: 73.9±3.1% and siRNA #693+Mela: 77.2±2.9%) in the COCs of TUDCA or melatonin treatment after pre-transfected siRNA #693. Additionally, we investigated the UPR marker proteins (BIP/GRP78, p-eIF2α, eIF2α, ATF4 and P90ATF6) in COCs of TUDCA or melatonin treatment after pre-transfected siRNA #693, respectively. As shown in the Fig. 2B and 2C, expression levels of most UPR marker proteins were significantly recovered in TUDCA or melatonin treated COCs after siRNA #693 transfection (p<0.001). These results suggested that induction of ER-stress by siRNA #693 for inhibition of BIP/GRP78 gene expression was affected the meiotic maturation in porcine COCs during IVM.
The table represents the % of oocytes in different stages of oocyte maturation in control and siRNA #693-transfected COCs with or without TUDCA (200 μM) and melatonin (0.1 μM) treatment. Data are represented as mean±SD of three independent experiments. a~d superscripts denote a significant difference among groups (p<0.05).
DISCUSSION
The purpose of the present study is to examine the effect of BIP/GRP78-knockdown (using siRNA #693) on ER stress and meiotic maturation in porcine oocytes during IVM. Previously, ER-molecular chaperones, GRP78 and GRP94, have been identified in the germinal vesicle (GV), germinal vesicle breakdown (GVBD), metaphase I (M I), and metaphase II (M II) stages of porcine oocytes, by 2-dimensional gel electrophoresis (2-DE) (Ellederova et al., 2004). The roles of ER stress or the regulation of UPR signaling mechanisms has been reported in mouse embryos. BIP/GRP78 gene is key regulator for ER stress response. In addition, ER stress regulation is involved in proliferation, differentiation and cellular apoptosis through the enhancement of UPR marker protein expression. However, there is no report on the directly role and expression of BIP/GRP78 in the in vitro maturated oocyte and cumulus cells oocyte complex (COCs) of porcine. As shown in Fig. 1B and 1C, protein levels of BIP/GRP78 were decreased in siRNA#693 treated COCs compared with negative siRNA and/or control group. These results suggest that BIP/ GRP 78 expression is correlated with the regulation ER-stress and UPR signaling pathways in COCs during IVM progression. Because of the importance of BIP/GRP78 in ER stress, we investigated the BIP/GRP78-mediated regulation of UPR signaling in porcine oocytes and its role in oocyte maturation.
BIP/GRP78 plays an important role in signal transduction via formation of different complexes with other proteins on the cell surface and in ER lumen (Mukherjee & Soto, 2011). BIP/GRP78 (binding immunoglobulin protein: BiP, also known as glucose-regulated protein: GRP78) is an endoplasmic reticulum (ER) chaperone protein, which is encoded by HSPA5 and contributes to protein folding in the ER compartment. Recently, many reports have shown that the BIP/GRP78 is involved in female reproduction system, oviduct, and early embryonic development. Previous report has shown that the maternal contribution of HSP90b1 (encodes GRP94) is critical for the development of murine zygotes and is involved in specific aspects of the first mitosis of murine zygotes (Audouard et al., 2011).
Various studies have shown that regulation of ER stress and UPR signaling play critical roles in maturation of oocytes and cumulus cells, and early embryonic development. The BIP/GRP78 (HSPA5) regulates the ER-stress-induced UPR signaling, which is essential for protecting the inner cell mass from apoptosis in early embryonic development of mouse (Luo & Mao, 2006). Chemical chaperones reduced ER stress in a mouse model of type-2 diabetes (Ozcan et al., 2006). In addition, ER stress in porcine COCs impaired pentraxin3 (PTX3) secretion from cumulus cells and delayed embryonic development during the blastocyst stage (Wu et al., 2012). Our result demonstrated that siRNA #693 against BIP/GRP78 inhibited the expression of UPR signal activators, ATF4 and P90ATF6, in porcine COCs during IVM (Fig. 1D). Further, PERK-mediated phosphorylation of eIF2α during ER stress represses new protein synthesis by blocking the initiation codon. Phosphorylated eIF2α (p-eIF2α) also induces expression of a transcription activator, ATF4, which regulates integrated stress response (Teske et al., 2011b). In the present study, we found that the PERK/p-eIF2α/ATF4 signaling pathway is downregulated in siRNA #693-transfected COCs (Fig. 2B and 2C).
Next, we hypothesized that if BIP/GRP78-regulated ER-stress delayed maturation of porcine oocytes, inhibition of ER stress should restore the maturation process. Melatonin is a well known oxidative stress scavenger (Agarwal et al., 2005) and has antioxidant properties (Poston et al., 2011). We used melatonin as an inhibitor of ER stress in porcine oocytes during maturation as described previously (Carloni et al., 2014). We also used TUDCA as another ER-stress inhibitor. As shown in the Table 1 and Fig. 2, TUDCA and melatonin treatment could restore the ER-stress-induced inhibition of meiotic maturation of porcine COCs. Concurrently, expression of UPR markers, including BIP/GRP78, was significantly increased in TUDCA and melatonin-treated COCs.
In conclusion, our results emphasized that BIP/GRP78 regulates ER stress during oocyte maturation, as well as plays key roles in in vitro maturation of porcine oocytes.

