INTRODUCTION
The reproductive strategy is unique from animal to animal. The Syrian hamsters follow the germ cell-forming process like rat and mouse investigated widely. But their reproductive activities are entirely arrested and become infertile in the winter climate of temperate zone (Choi & Lee, 2012; Choi, 2013). The seasonal changes of reproductive function have been well established to be regulated by photoperiod (Stetson & Watson-Whitmyre, 1984; Stetson & Watson-Whitmyre, 1986). Long photoperiod as in summer (LP, equal to or greater than 12.5 hours of lightings in a day) sustains large testes but short photoperiod of winter (SP, equal to or less than 12 hours of lightings in a day) reduces discernibly the size of testes. Thus spermatogenesis is vigorously developed in LP and ceased completely in SP (Choi & Han, 2010; Choi & Lee, 2012).
The natural changes of seasonal reproductive function in the hamsters can be recapitulated in the laboratory lighting regime (Stetson & Watson-Whitmyre, 1984). When the sexually mature male hamsters are moved to SP, they lose reproductive activities in 8 full weeks, showing reduced testicular mass and no spermatozoa. If the length of lighting in a day is set to LP mimicking summer daylight, the sexual functions are obviously resumed and recovered to LP conditions.
The spermatozoa are produced in the testes from where the haploid spermatozoa begins to travel male reproductive track. On the course of the journey they meet the substances secreted from male reproductive organs and obtain capacity to fertilize the eggs (Nixon et al., 2006; de Lamirande, 2007). In the post-testicular maturation process they are exposed to various substances secreting from the Sertoli cells, epididymis, vas deferens, seminal vesicle, and prostate cells. The surface membrane of the spermatozoa undergoes diverse modification during which they are protected from the threat of microorganisms and reconstructed to gain fertilization capacity (Cohen et al., 2001; Dacheux et al., 2003; Bourgeon et al., 2004; Jalkanen et al., 2006; Nixon et al., 2006; de Lamirande, 2007).
One of the accessory reproductive organs is the seminal vesicles (vesicular glands or seminal glands) that are a pair of simple tubular glands postero-inferior to the urinary bladder of mammals (Hendry et al., 2006; Vidigal et al., 2010). They secrete a significant proportion of the fluid that ultimately becomes semen. One of the components released from the seminal vesicles is the semenogelin (SEMG). It has been reported to bind to the epididymal protease inhibitor (EPPIN) that is synthesized and secreted from the Sertoli cells of the testes and epididymal epithelial cells (Sivashanmugam et al., 2003; Bian et al., 2009). The complex that the EPPIN anatomically produced in advance combines with SEMG (EPPIN-SEMG complex) protects the spermatozoa from the microbial attacks (Yenugu et al., 2004; de Lamirande, 2007). Further the complexes are degraded by prostate specific antigen (PSA) enzyme that results in the production of fragments of SEMG, contributing the lighter spermatozoa to increase the progressing motility in order to approach to the ovulated egg (O’Rand et al., 2006). On the other hand, there are other proteins clusterin and lactotransferrin bound to spermatozoa already coated with EPPIN (Paasch et al., 2011; Zhang et al., 2013). Such a function of SEMG is important to mammalian reproduction and evoluted phylogenetically. However, the informations in Syrian hamsters is very limited.
The ejaculated spermatozoa are veneered with SEMG and undergo a temporary retardation of forward motility, resulting in infertility (O’Rand et al., 2004; O’Rand et al., 2009; Mitra et al., 2010). The progressing motility is resumed at the time when the PSA works for the degradation of the complex. Persistent SEMG on the surface of spermatozoa draws the seminal hyperviscosity and infertility (Esfandiari et al., 2008; Emami et al., 2009; Du Plessis et al., 2013). Moreover, it was documented that the presence of SEMG and EPPIN increased the frequency of idiopathic male infertility in specific population of human, thus proposing utilization of them to identify human male infertility (Wu et al., 2019). In in vivo fertilization, SEMGs inhibited ectopic capacitation before sperm reach the fertilization site and the number of total SEMG-unbound sperm was directly linked to the possibility of fertilization (Yamasaki et al., 2017).
The goal of the present work was to identify the SEMG gene, compare to genes reported in other rodents, and to correlate the SEMG gene with reproductive capability of male Syrian hamsters maintained in different photoperiod.
MATERIALS AND METHODS
Male Syrian hamsters (Mesocricatus auratus) were used to identify the SEMG gene. They were housed in plastic cages under LP conditions of light and dark (lights of 14 h:darkness of 10 h) with an ambient temperature of 22±1°C. Reproductive activities of these hamsters in 8 weeks after birth are always active in the photoperiod. Spermatozoa were confirmed in the testis and the epididymis by the microscopic examination. The animals were fed with standard laboratory mouse chow and tap water ad libitum. Some of the reproductively mature Syrian hamsters were transferred to SP (lights of 10 h:darkness of 14 h) and kept for 8 weeks during which their reproductive activity is entirely degenerated. The seminal vesicle tissues among the reproductive organs were extracted and immediately subjected to the reverse transcription polymerase chain reaction (RT-PCR). The condition of management of animals was approved by the Yongin University Institutional Animal Care and Use Committee (YUIACUC-2018-02).
The expressions of SEMG gene were examined in seminal vesicles of the hamsters who had active and inactive reproduction capacity by regulating the photoperiod.
The male Syrian hamsters were sacrificed by decapitation. Immediately the testes were excised and the suitable parts of them were immersed in the physiological saline. The tissues were cut many times with sterile scissors without delay. Following 1 min at room temperature, 1 mL of supernatant was transferred into microcentrifuge tube. The tube was spun at 14,000 rpm for 1 min (CentrisartⓇA-14C, Sartorius, Germany). The supernatant was removed and 1 mL of saline was added to the tube. The pellet in the tube was dispersed by sucking and releasing of pipette. One drop of the suspension was fallen onto the slide glass and smeared uniformly. Then the slide glass was dried completely and absolute methanol was fully applied. After the entire dryness of the slide, specific staining solution was employed and dispersed. Ten minutes later, the extra staining solution was cleanly washed with flowing tap water. Then the presence of spermatozoa was observed by light microscope (Leica DM500, Leica Microsystems, Switzerland).
The SEMG gene of Syrian hamster has not been identified up to now. Thus primer sequences for SEMG gene of the animal were chosen from the Rattus norvegicus SEMG 1 mRNA sequence (NM_012710.2) and the Mus musculus seminal vesicle secretory protein 2 mRNA (NM_017390.4) in the NCBI Reference Sequences. Also the mRNA from the Chinese hamsters was referenced (predicted mRNA of Cricetulus griseus SEMG I, NCBI Reference Sequence (XM_003504573.1). The primers selected were 5’-tggccaacaaaaatccct-3’ for forward direction and 5’-ctgcccctccctttgtaa
aa-3’ for reverse direction. The anticipated size was 302 bp. The primers have high homology in comparison to the sequences of mouse and rat. Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) PCR was used as reference standard for RT-PCRs in the present study. The primers of GAPDH were 5’-aaatgaccccttcattgacc-3’ for forward and 5’-ccttccacaatgccaaagtt-3’ for reverse. The anticipated size was 420 bp. Sequence analyses were done by a commercial sequencing service company (Bioneer, Korea).
Total RNAs were isolated from tissue samples using TRIzolⓇ Reagent (Invitrogen, USA) according to the manufacturer’s protocol. That is, the small pieces of tissues (50-100mg) were excised and subjected to sonicate with 1 mL of TRIzolⓇ Reagent (VCX130, Vibra CellTM, Sonics & Materials Inc., USA). The samples were transferred to new microcentrifuge tubes and spun for 5 min at 12,000 rpm at 4°C. The supernatant was moved into the new tubes and left for 5 min of incubation, allowing to permit complete dissociation of the nucleoprotein complex. 0.2 mL of chloroform was added and capped firmly the tubes. Following the incubation of 2-3 min, the tubes were spun for 15 min at 12,000 rpm at 4°C. The upper aqueous phase was transferred to the new tubes. Half mL of isopropanol was added and incubated for 10 min. Then the tubes were spun for 10 min 12,000 rpm at 4°C. The supernatant was discarded and the pellets were resuspended in 1 mL of 75% ethyl alcohol. After agitation, the samples were spun for 5 min at 7,500 rpm at 4°C. The supernatant was eliminated and the pellets were allowed to dry for at least 5 min. The pellet was solubilized with 20-50 μL of RNase-free water. Quantitation of the RNA was measured by the absorbance at 260 nm that provides total nucleic acid content and 280 nm that determines purity of the RNA.
The extracted RNAs were used in RT-PCR reactions carried out with Maxime™ RT PreMix and AccuPower PCR Premix (Bioneer, Korea) according to the manufacturer’s instructions. Reverse transcription was primarily carried out to create complementary DNAs (cDNAs) representing cell-specific RNA populations. The proper amount (1 pg-1 μg) of tRNA was transferred to clean microcentrifuge tubes and mixed with the following materials: DEPC-treated water, reverse transcription reaction buffer, oligo (dT)20 primer, dNTPS (dATP, dTTP, dCTP, dGTP), reverse transcriptase, and RNase inhibitor. The tubes were gently agitated and incubated at 42°C for 60-90 min. In order to inactivate the reverse transcriptase the tubes were heated to 85°C for 5 min. The cDNA products transcribed were stored at –20°C.
PCR was performed with the cDNA diluted with TE buffer (10 mM Tris (pH 8.0), 0.1 mM EDTA). The microcentrifuge tubes with template cDNA (typically 10 ng) were mixed with water, 10× PCR Buffer, dNTP Mix, primers (forward and reverse), Taq DNA Polymerase, and 25 mM MgCl2. The tubes were stirred gently by vortexing and spun briefly to collect all components to the bottom of the tubes. The cycles of PCR were 40 with repeating the following in the order: denaturing temperature of 94°C for 20 seconds, annealing temperature of 55°C for 30 seconds, and extension temperature of 72°C for 1 min. The final extension was performed at 72°C for 5 min and then cooled down to 4°C.
The reaction products were analyzed by gel electrophoresis in 1.5% agarose gel (100 V, 60 min) and visualized by ethidium bromide staining. The bands were identified using the image analysis system (Chemi Doc XRS, Bio-Rad, USA).
The PCR products were purified through the agarose gel electrophoresis according to the manufacturer (AccuPrepⓇ PCR/Gel Purification Kit, Bioneer Corporation, Korea). The PCR products were subjected to the electrophoresis and stained with ethidium bromide. The visualized gel bands were cut out using blade. The gel slices were mixed with 3 volumes of FB buffer. The tubes were incubated at 50°C for 10 min with mixing by inverting every 2-3 min. One volume of absolute isopropanol was added and mixed immediately by inverting. The mixture was transferred to a binding column in a 2 mL collection tube. The lid was closed and spun at 14,000 rpm for 1 min. The binding column was reassembled with collection tube after removing the flow-through fluid. 500 μL of W2 buffer was added and spun at 14,000 rpm for 1 min. The binding column was reassembled like above. Then the step with W2 buffer was repeated once more and spun at 14,000 rpm for 1 min. The binding column tube was transferred to a new 1.5 mL tube for elution. 30 μL of EA buffer was added carefully onto the binding column tube and waited for at least 1 min at room temperature. Finally the new tube was spun at 14,000 rpm for 1 min. The eluant was sent to Bioneer (Korea) to analyze the sequence of SEMG gene.
RESULTS
The male Syrian hamsters housed in LP for 8 weeks showed large testes that was 1.4g of average of each testis, which represents full spermatogenesis. The expression of SEMG gene was primarily detected in the seminal vesicles of male Syrian hamsters (Fig. 1). The size of the SEMG gene sequenced was 308 bp, which is 6 bp longer than expected (Fig. 2). The seminal vesicles of males obviously showed the expression of SEMG. GAPDH was used as reference standard for RT-PCRs.
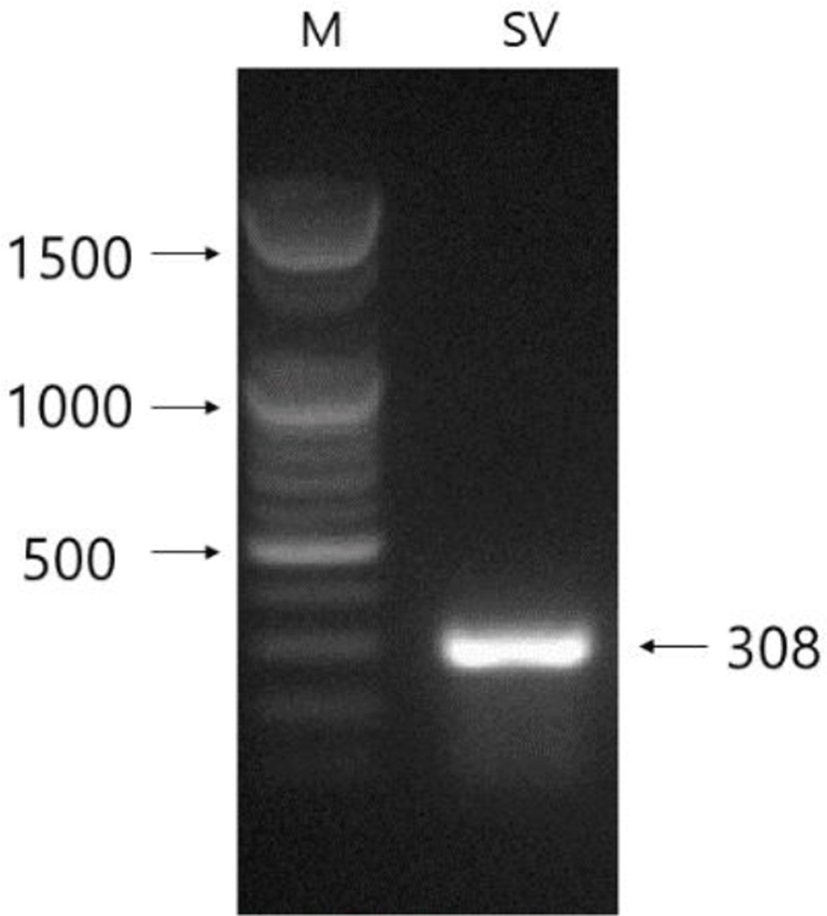

The mRNA of SEMG gene of Syrian hamster that identified in this investigation was compared to other SEMG genes of chinese hamster, rat, and mouse reported in the range of sequence detected (308 bp, Fig. 2). The sequence of SEMG of the Syrian hamster had homology of 94.8% to the predicted sequence of Chinese hamster (Cricetulus griseus) SEMG1 mRNA (XM_003504573.1), 85.1% to Rattus norvegicus SEMG1 mRNA (NM_012710.2), and 84.4% to mouse (NM_017390.4). A point to be worth noticing is that there are gaps between the nucleic acid sequence of SEMG gene of Syrian hamsters and those of other rodents. Particularly, there were gaps of 3 places in the comparison to the chinese hamster. In the matching of the sequence of SEMG gene of Syrian hamsters to that of other animals in order to attain the best correspondence, there were discrete gaps of several places.
In order to inspect the relationship of SEMG and fertility ability in male Syrian hamsters, the expression of SEMG gene was investigated in the two functionally different testes: reproductively energetic testes and completely regressed testes of the Syrian hamsters maintained in LP and SP, respectively. The Syrian hamsters housed in LP had large testes of 2.1±0.03g of mean weights of testes and the animals maintained in SP for 8 weeks showed very small testes of 0.4±0.02 g of mean weights of testes. The representative mass of testis in each photoperiod was shown in Fig. 3A. In the histological view of testes, LP animals had all kinds of germ cells but SP animals primarily spermatogonia and some spermatocytes (Fig. 3B). The seminal vesicles also showed similar aspects in which LP hamsters demonstrated relatively bigger and heavier seminal vesicles than those of SP animals (0.8±0.01 g in LP vs 0.3±0.03 g in SP, Fig. 3C). The LP animals had abundant spermatozoa in testis but the SP animals showed no spermatozoa at all in the organ tissue (Fig. 3D).
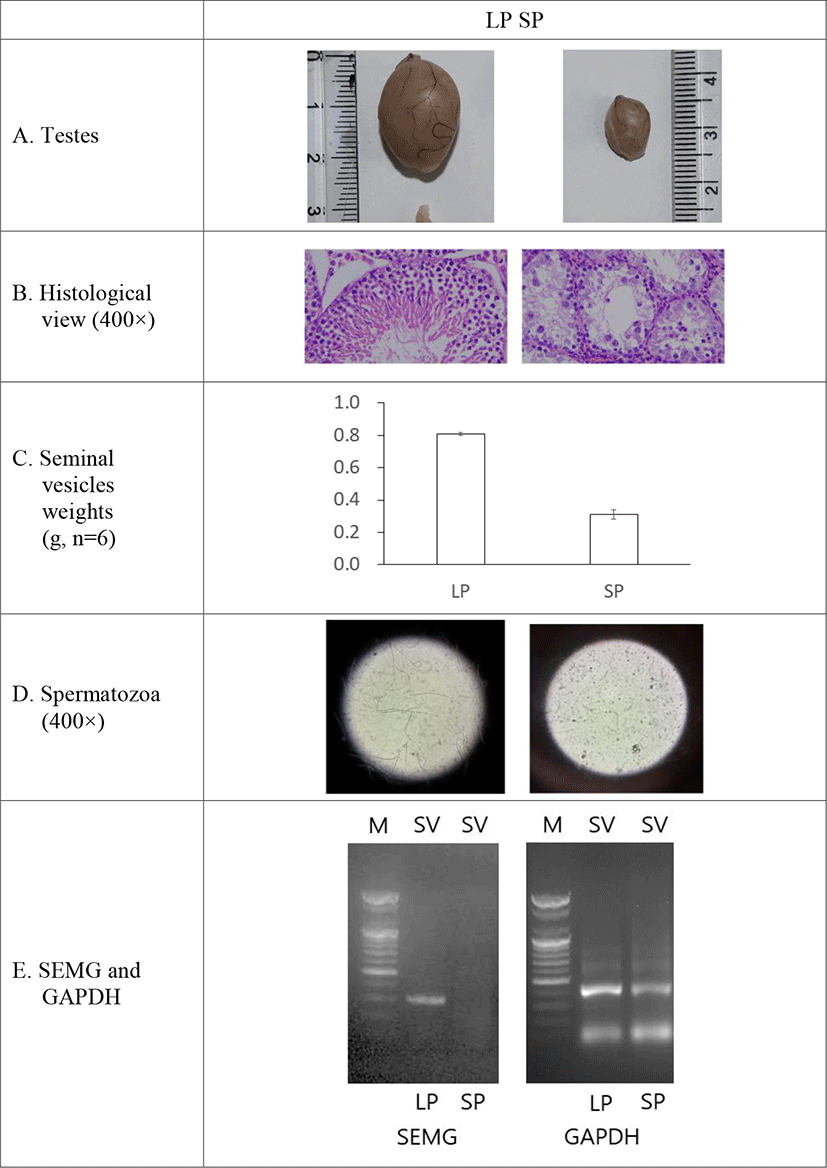
The expression of SEMG gene was witnessed in the seminal vesicles of the reproductively active Syrian hamsters kept in LP (Fig. 1). The gene was not detected in the SP-induced small testes, which is an indicative of cessation of spermatogenesis to be ended (Fig. 3E).
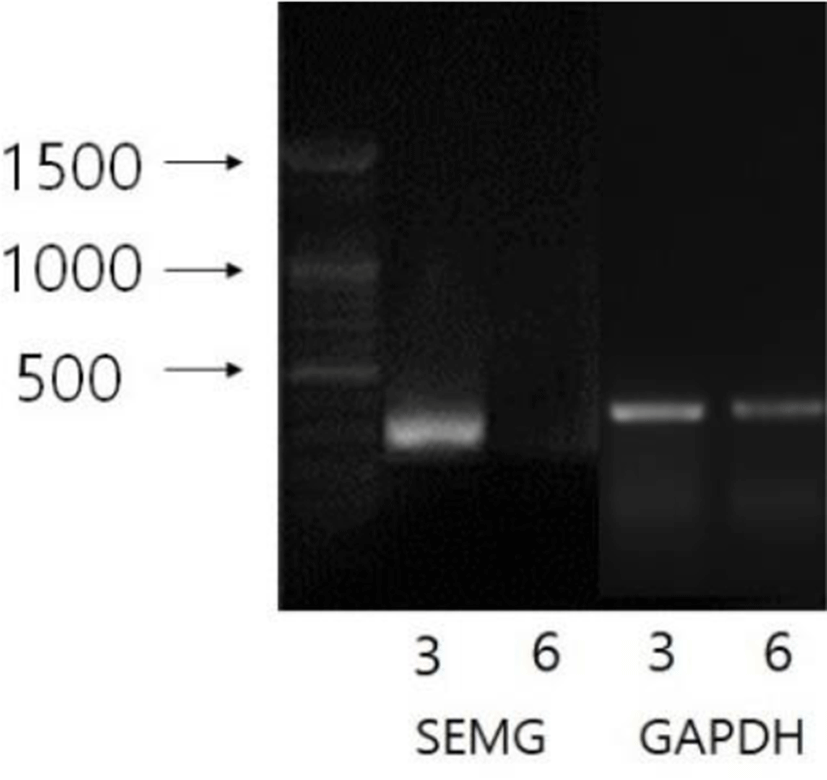
The expression of SEMG gene was examined in the seminal vesicles from the period of time after maintaining the Syrian hamsters in SP. During the course of SP they were subjected to RT-PCR at the indicated time points. The expressions of the gene in the seminal vesicles were shown in Fig. 4. The SEMG mRNA was detected at the third week of SP, blurred at the sixth week of SP, and ultimately vanished at eighth week at which this experiment was planned to be ended. The results were compatible with the fertile ability of male Syrian hamsters housed in SP.
DISCUSSION
The present results represent the correlation of the SEMG gene with the fertility capability in male Syrian hamsters. Only reproductively active Syrian hamsters expressed SEMG genes in seminal vesicles. In the sexually regressed testis of SP animals, SEMG mRNA was not detected in seminal vesicles. And the sharply reduced detection of SEMG mRNA was emerged at the 6th week in the male Syrian hamsters housed in SP. This is the first report that the expression of SEMG gene is directly correlated to the fertility competence in these animals.
The Syrian hamsters exhibit completely regressed testes in 8 weeks of SP in laboratory facility (Stetson & Watson-Whitmyre, 1984; Stetson & Watson-Whitmyre, 1986). The degenerated testes are confirmed by relatively shortened diameter of more than one half of seminiferous tubule (nearly one eighth in volume), indicating the absence of germ cells experiencing meiosis (spermatozoa, spermatid, and spermatocytes) in the histological examination (Choi, 2013).
In the present research, the SEMG gene was expressed in seminal vesicles of the sexually active male Syrian hamster. These results were previousy observed and some other tissues were also reported to express the gene (Lundwall et al., 2002). They described SEMG as the predominant human seminal plasma proteins to be expressed in non-genital tissues, meaning other funtions unknown so far.
When the SEMG gene identified in male Syrian hamster was compared to the genes of other species (Fig. 2), it had very high proportion of homology, impling the same function mentioned in other reports (Richardson et al., 2001; Sivashanmugam et al., 2003). The sequence of SEMG gene of the Syrian hamster identified in this investigation had broad homology to that of other rodents (94.8% to Chinese hamster, 85.1% to rat, and 84.8% to mouse). The comparable similarity denotes a possible role reported previously.
The SEMG protein provides a basis to reduce spermatozoa motility, leading to prevention of the spermatozoa to fertilize the egg (O’Rand et al., 2011; O’Rand et al., 2016; O’Rand et al., 2018). The antibody to the SEMG protein also raise a possibility as a contraceptive by inhibiting the action of the subsequent protein in the pathway of the spermatozoa to gain fertilization capability (O’rand et al., 2004). Thus the gene product presents a notable site to develop an anticonceptive agent.
In the male Syrian hamsters whose sexual activity was completely arrested by SP, the SEMG gene was not expressed in seminal vesicles. The distinguished regression was assured by the reduced size, lowered weights of testes, and the absence of spermatozoa (Choi & Lee, 2012). Accordingly, the results demonstrate that the SEMG gene could be a possible (semen) biomarker because the gene is exclusively expressed in the reproductively energetic animals (Suttipasit & Wongwittayapanich 2018).
On the other hand, the function of SEMG protein mentioned above raises a reasonable possibility that a natural product would exert as a contraceptive agent if it binds to the SEMG protein on the surface of spermatozoa. SEMG protein can be bound by another protein SEMG, which provide a reaction site for PSA, resulting in the increase of spermatozoa motility. By the binding of a natural product to the SEMG, if SEMG can not bind to the SEMG and PSA is unable to recognize the SEMG-SEMG complex, the capability of the spermatozoa to fertilize the egg will be sharply reduced. The incapability will be appeared by increase of viscosity, reduction of motility, and inhibition of action of PSA.
It has not been established when the expression of the SEMG gene is inhibited in the course of SP environment. The expression of SEMG is likely to be diminished at the 6th week of SP. A factor (or factors) that prevent the SEMG gene from expressing in the reproductive organs also has not been known at this time. The inquiry remains to be investigated.
In conclusion, male Syrian hamsters expressed SEMG genes in seminal vesicles. In the reproductively regressed testis of SP animals, SEMG mRNA was not detected, suggesting that the expression of the SEMG gene is associated with fertility capability in male Syrian hamsters. It is a future work when and how the expression of the SEMG gene is controlled in the males housed in SP.

