INTRODUCTION
Studies on the reproductive cycle, sexual maturation, and spawning of Haliotidae species have been conducted to accumulate data on basic biology, resource management, and culture technology development (Webber & Giese, 1969; Sobhon et al., 1999; Najmudeen & Victor, 2004; Visser-Roux, 2011).
In Korea, two species and two subspecies of the genus Haliotis, and two subspecies of the genus Sulculus have been reported (Lee et al., 2015). Research on their reproduction was mostly undertaken using H. discus hannai (Lee, 1974; Rho & Park, 1975; Kim et al., 2016), whereas studies on H. gigantea have rarely been conducted.
H. gigantea is an edible gastropod with a shorter shell height than that of the other Haliotidae found in Korea, and it also shows relatively rapid growth. In Korea, this species is found mainly on the southern coast and on the coast of Jeju Island, which have relatively high water temperature (WT). This gastropod inhabits rocky bottom in intertidal zones 30 m underwater (Lee et al., 2015). This study aimed to obtain the reproductive biological data (sex ratio, gonadal development, reproductive cycle, main spawning season etc.) of H. gigantea from Jeju Island, Korea.
MATERIALS AND METHODS
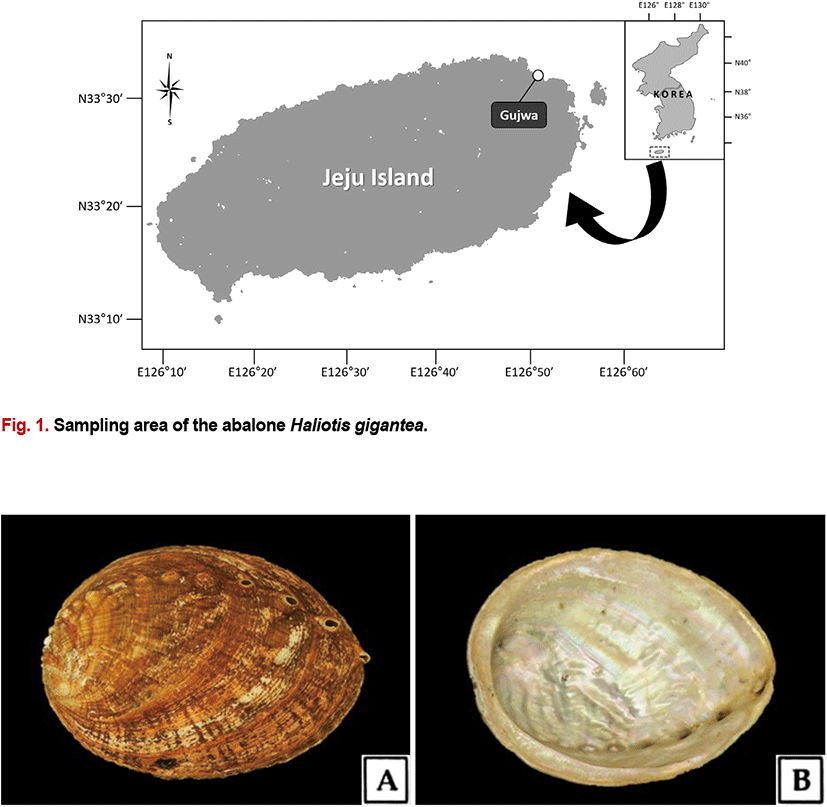
H. gigantea specimens were collected by divers from the coast of Gujwa-eup, Jeju Island in Korea (Fig. 1). Specimens were collected monthly from January to December 2014, and a total of 290 specimens were collected for the study. The specimens analyzed had a shell length of 111.2 (±13.3) mm and a total weight of 136.1 (±55.8) g. Morphological characteristics are shown in Fig. 2.
The data of monthly WT in the sampling area were referenced from the Korea Hydrographic and Oceanographic Administration (KHOA, 2014).
Specimens were dissected for light microscopy analysis after their morphometric characteristics (i.e., shell length, total weight, and body weight) had been measured. The hepatopancreas including the gonads was fixed in Bouin’s solution for 24 h, rinsed with running water for 36–48 h, and dehydrated through a graded ethyl alcohol series (70%–100%). The specimens were then embedded in Paraplast (Leica, Germany), following which they were sectioned into slices of 4–6 μm thickness using a microtome (RM2235, Leica, Germany). The tissue specimens were then stained with Mayer’s hematoxylin–0.5% eosin (H–E) and Masson’s trichrome stain. An image analyzer (IMT-VT Image analysis, IMT, USA) was used to quantify the size of the oocytes.
The sex ratio and the proportion of females were computed with the following equations:
The gonad index (GI) was corrected, in part, using the method described by Eversole (1997). Gonad development was categorized into the following six stages: inactive (In), early active (Ea), late active (La), ripe (R), spent (Sp) and degenerative stage (D). Samples were quantified by multiplying each individual with a constant (In, 1; Ea, 2; La, 3; R, 4; Sp, 3; D, 2) for each gonadal development stage.
The meat weight index (MWI) and condition index (CI) and were calculated with the following equations: MWI = [Body weight (g) / Total weight (g)] × 100; CI = [Body weight (g) / Shell length (mm)3] × 1,000.
The sex ratio was analyzed using SPSS 21.0 software (SPSS, USA). The observed female:male ratio for each shell length group was compared with the expected ratio of 1:1 using the chi-square (χ2) test. Differences were considered statistically significant at a p-value of less than 0.05.
RESULTS
The average WT at the sampling area of H. gigantea during the sampling period was 17.2°C, with the lowest temperature in January being 12.2°C and the highest in September being 23.1°C.
In the histological analysis of the gonads, simultaneous hermaphroditism was not observed in H. gigantea. The average sex ratio (female:male) was 1:1.74, where the female proportion (F/F+M) of the 290 specimens was 36.6%, indicating a distinctly higher proportion of males (Table 1).
The gonad of H. gigantea comprised many gametogenic follicles and showed distinct seasonal differences, as outlined in the next two subsections.
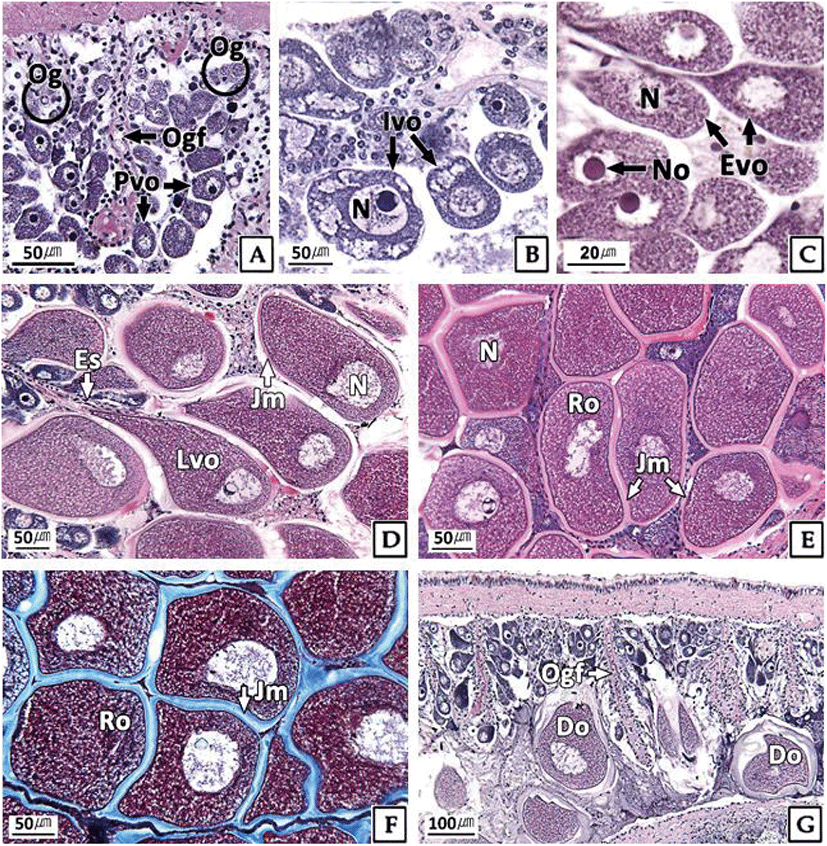
The developmental stages of ovary showed seasonal differences. At the inactive stage, the ovary was filled with mainly oogonia (diameter 10–15 μm) and previtellogenic oocytes (diameter about 20 μm) (Fig. 3A). During January and February, 100% of females were in the inactive stage, and more than 50% remained in this stage for a relatively long period (from January to July) (Fig. 5).
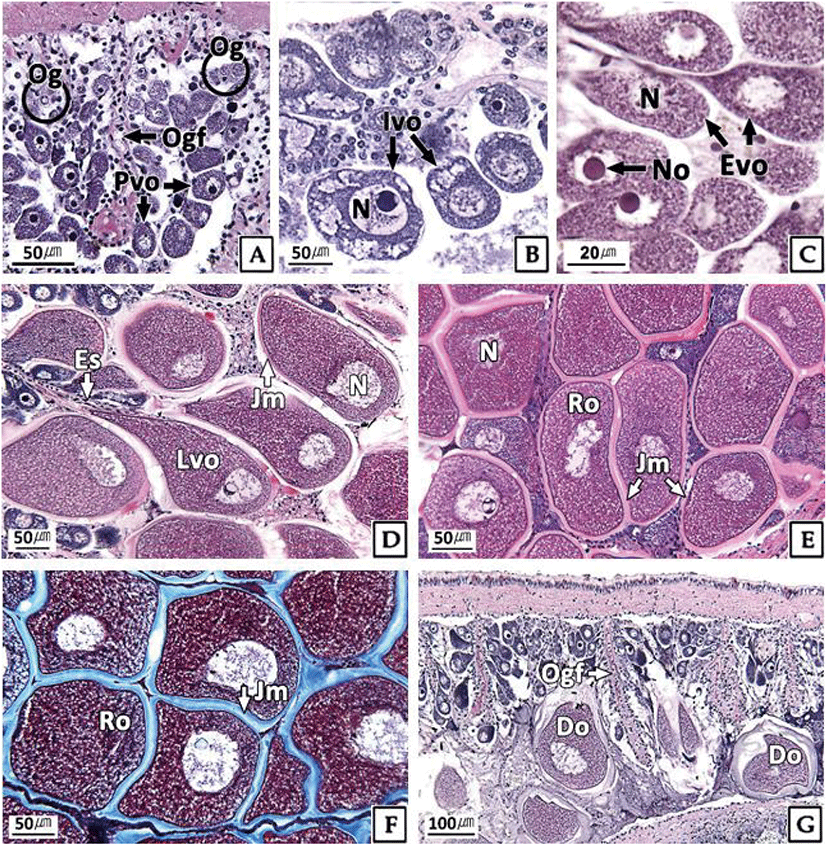
The ovaries at the early active stage were found mainly from March to September, where initial vitellogenic oocytes (diameter about 30 μm) (Fig. 3B) and early active vitellogenic oocytes (diameter about 65 μm) of oval shape were mainly observed (Fig. 3C). A high percentage of females in the late active stage occurred mainly in August and September (Fig. 5). During this period, the ovaries were filled mainly with late active vitellogenic oocytes but did have some early active vitellogenic oocytes. The late active vitellogenic oocytes were rectangular in shape, having a distinct egg stalk connected to the oogenic follicle, which was approximately 160 μm in diameter (Fig. 3D). In October, the percentage of females in the ripe stage was 46%, which was the highest percentage observed during the year. The ovaries were filled mainly with ripe oocytes of rounded polygonal shape with a well-developed jelly membrane and an average diameter of approximately 200 μm (Fig. 3E, F). Ovaries in the spent and degenerative stages were observed mainly from October to December. During this period, the ovarian tissue showed the degradation and absorption of undischarged oocytes after spawning (Fig. 3G).
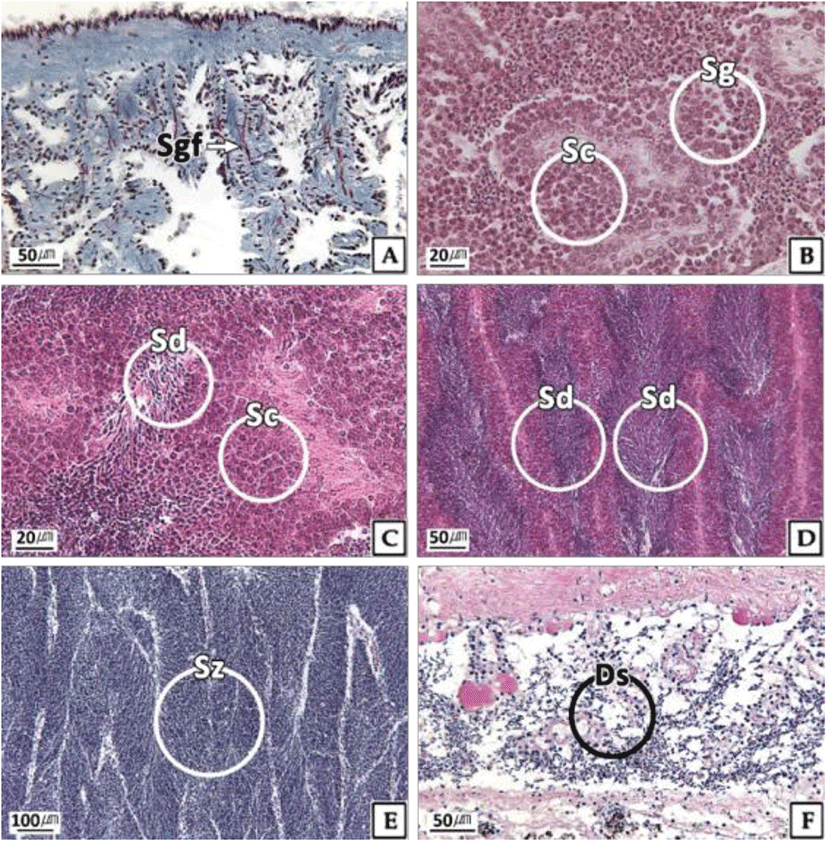
The testicular development stages of H. gigantea also showed seasonal changes. At the inactive stage, spermatogonia were observed in parts of testicular tissue, but the proportion of empty space was high owing to the undeveloped germ cells (Fig. 4A). During January and February, 100% of males were in the inactive stage, and more than 50% remained in this stage during the relatively long period from January to July (Fig. 5). Testes at the early active stage were found mainly from March to September, where mostly spermatogonia and spermatocytes and a small number of spermatids were observed (Fig. 4B, C). A high percentage of the males were in the late active stage during August and September, during which the testes were filled mostly with basophilic spermatids but had some spermatocytes present (Fig. 4D). In October, the percentage of males in the ripe stage was 46%, which was the highest percentage observed during the year, and the testis was filled mainly with basophilic sperm (Fig. 4E). Testes at the spent and degenerative stages were observed mainly from October to December, where the testicular tissue showed degradation and absorption of remaining sperm after the spent (Fig. 4F).
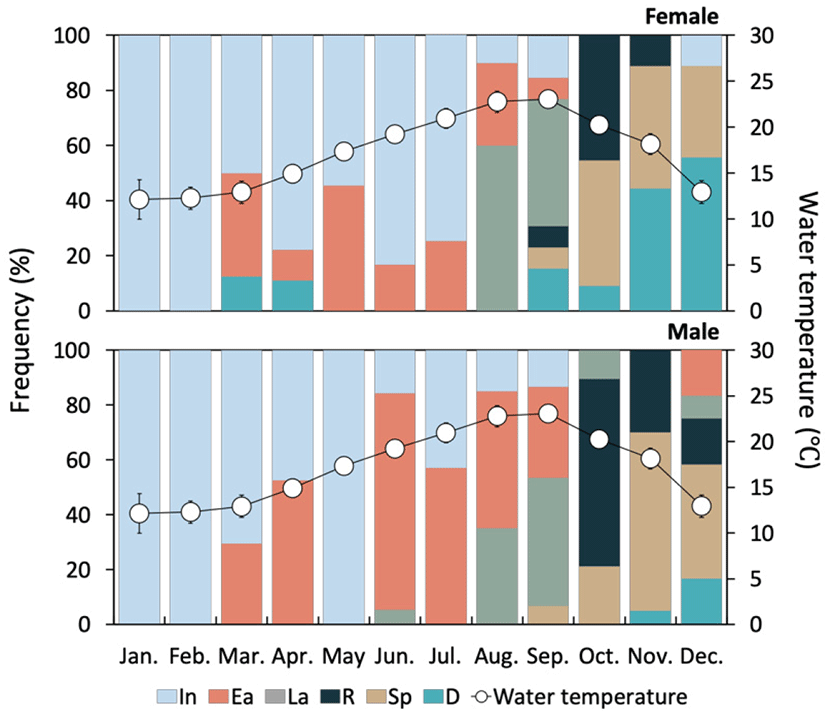
Similar to the gonad developmental stages, the GI changes in both males and females showed distinct seasonal patterns; namely, an increase in fall, and a decrease in spring and winter. The GI began to increase in March (1.36) and was the highest in October (3.57), and then decreased in November to less than 3.0 (Fig. 6).
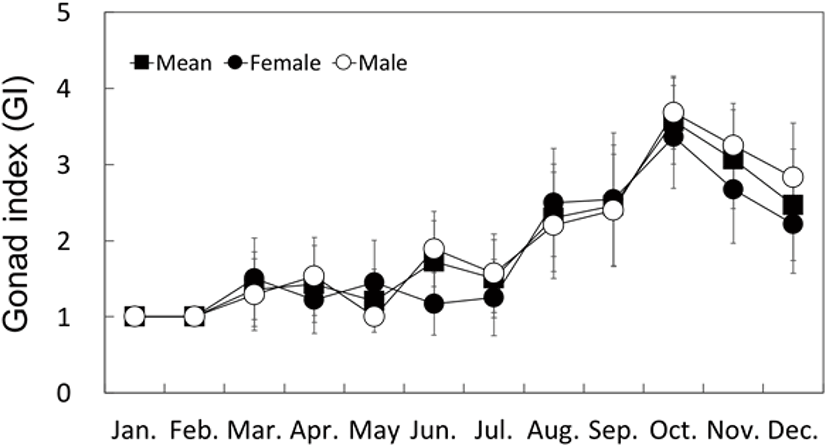
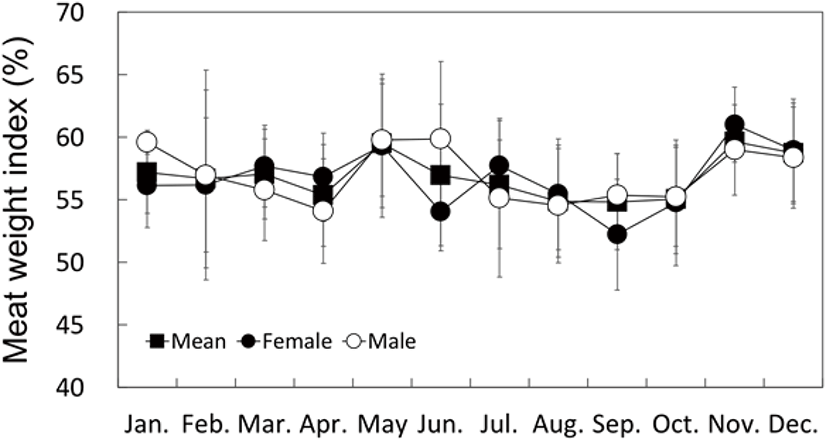
Although the MWI and CI for May and November displayed higher values than those at the other times of the year, they did not display definitive seasonal changes as observed with the GI (Figs. 7 and 8).
On the basis of the monthly changes in gonadal development stage and the GI, the reproductive cycle could be categorized into inactive ( January to May), early active (May to July), late active (August to September), ripe (October), spent (October to November) and degenerative stage (November to December).
DISCUSSION
The sex ratio (female:male) of Haliotidae was reported to be approximately 1:1 in Haliotis cracheroidii (Webber & Giese, 1969), H. rufescens (Young, 1970), H. australis (Wilson & Schiel, 1995), H. asinina (Capinpin et al., 1998), and H. varia (Najmudeen & Victor, 2004). The sex ratio of H. discus hannai collected from 3 regions in Korea by our group was approximately 1:0.9 on average, showing a slightly higher proportion of females, although not significantly different from 1:1 (Kim et al., 2016).
The sex ratio of H. gigantea was 1:1.74 (female:male), showing a lower proportion of females (approximately 36.6%, n=106/290). In December, which represents the inactive stage of H. gigantea, the sex ratio was 1:2.0, and the proportion of females was 33.3%. As abalones can be divided into males and females in the inactive stage after spawning, the low proportion of females was not a consequence of spawning. Further research is needed to investigate why the proportion of females in H. gigantea is lower than that in other abalone species.
The morphological expression of sex and changes in the sex ratio in molluscs are affected by genetic factors, age and environmental factors such as the WT and food (Yusa, 2007; Chávez-Villalba et al., 2011; Park et al., 2012). Therefore, there is a need for more detailed research in the future to identify the reason for the lower proportion of H. gigantea females.
The diameter of the ripe oocytes differs depending on the species within the same genus in the Haliotidae, as reported for the following species in various countries: 80–140 μm for H. asinina in Thailand (Sobhon et al., 1999), in excess of 125 μm for H. asinina in Panagatan Cays, Philippines (Capinpin, 1998), 180±20 μm for H. varis in the Gulf of Mannar in India (Najmudeen & Victor, 2004), 202±21 μm for H. discus hannai in Korea (Kim et al., 2020), 250–300 μm for H. midae in South Africa (Visser-Roux, 2011), and 250 μm for H. rufescens in California (Giorgi & DeMartini, 1977).
In this study, the diameter of the ripe oocytes of H. gigantea was measured to be approximately 200 μm. This suggests that the size variation of ripe oocytes of haliotids could be due to the extent of yolk accumulation. However, there is a need to investigate whether the extent of yolk accumulation is due to a species-specific characteristic or an ecological factor.
The WT is a major environmental factor that determines the sexual maturity of many molluscs (Mackie, 1984; Eversole, 2001; Grubert & Ritar, 2004). In general, the reproductive period of invertebrates that thrive in low-latitude regions is long, whereas that of invertebrates in high-latitude regions is short. These characteristics are due to the effects of different WTs on the reproductive characteristics of the species (Fretter & Graham, 1964).
On the bases of the monthly changes in gonadal development stages and the GI, the reproductive cycle of H. gigantea could be categorized distinctly into the inactive (January to May, WT 12.2°C–17.3°C), early active (May to July, WT 17.3°C–20.9°C), late active (August to September, WT 22.8°C–23.1°C), ripe (October, WT 20.3°C), spent (October to November, WT 20.3°C–18.1°C), and degenerative stages (November to December, WT 18.1°C–12.9°C). Based on these results, H. gigantea has one spawning season per year, in the fall, and its sexual maturation and reproductive cycles are affected by the WT.
From the data of reproductive cycle analysis, the gonadal development stages and GI changes are used to identify the period needed for gonadal maturation and the maturation speed of the species. Of the H. discus hannai specimens collected in the same region as H. gigantea, over 40% of the population were found to be at the inactive stage, which was from September to February for the females and from December to February for the males (Kim et al., 2016).
However, the periods with over 50% of H. gigantea at the inactive stage were from January to July for the females and from January to May for the males, which were longer than those for H. discus hannai. The periods in which there were a high proportion of individuals in the early active stage lasted approximately 3 and 4 months for H. discus hannai females and males, respectively, but were again longer (at approximately 6 months) for both the H. gigantea females and males. The late active stage lasted 2 months for H. gigantea, whereas it was 3 months for H. discus hannai. Furthermore, the GI increase gradient of H. discus hannai after the inactive and early active stages was relatively gradual compared with that of H. gigantea. These results showed that the reproductive characteristics of H. gigantea included longer inactive and early active stages, and shorter late active and ripe stages, relative to those of H. discus hannai. In addition, such reproductive characteristics could contribute to the difference in the ripe oocyte size.
The monthly change in the CI of bivalves has been shown to be correlated to their reproductive cycle (Park et al., 2003; Gosling, 2004; Marroquin-Mora & Rice, 2008). However, it is difficult to find research studies on the correlation between the CI change and the reproductive cycle in the Haliotidae. In this study, the MWI and CI of H. gigantea did not show definitive seasonal changes like those of H. discus hannai (Kim et al., 2016).