INTRODUCTION
Kisspeptin is a peptide translated from the Kiss gene. Following the identification of the Kiss gene, various studies to clarify the function of kisspeptin suggested that it stimulates gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus to promote the secretion of gonadotropin hormone (GtH) (Parhar et al., 2004). However, kisspeptin is expressed in tissues other than the hypothalamus in fish (Oakley et al., 2009), and kisspeptin expression in the pituitary may directly affect reproduction in mammals (Gutiérrez-Pascual et al., 2007; Richard et al., 2008). The Kiss1 and Kiss1 receptor genes are expressed in gonadotrophs in the pituitaries of rats, and their expression can be controlled by oestradiol and GnRH (Richard et al., 2008).
Although the kisspeptin gene is expressed in the pituitaries of several species of fish and mammals (Escobar et al., 2013; Lehman et al., 2010; Ogawa & Parhar, 2013; Park et al., 2016), the function of kisspeptin in the pituitary is not clear (Navarro et al., 2005; Smith et al., 2008; Suzuki et al., 2008). Treating the pituitary cells of goldfish (Carassius auratus) with kisspeptin1 stimulated the secretion of luteinizing hormone (LH; Yang et al., 2010), although kisspeptin2 did not affect LH secretion (Li et al., 2009). A direct effect of kisspeptin on the pituitary was observed in the European eel (Anguilla anguilla); treatment of eel pituitary cells with lamprey (Petromyzon marinus) or zebrafish (Danio rerio) kisspeptin peptides reduced luteinizing hormone β subunit (lhβ) mRNA expression (Pasquier et al., 2011). These experimental results suggest that kisspeptin has a direct, species-specific function in the pituitary.
The pituitary of sexually mature male and female Nile tilapia (Oreochromis niloticus) also exhibited elevated expression of the Kiss2 gene (Ogawa et al., 2013) suggesting a direct or indirect role for kisspeptin in reproduction at the pituitary level. However, the direct effect of kisspeptin on the pituitary of Nile tilapia has not been investigated. Therefore, the present study was performed to investigate the effects of various concentrations of synthetic kisspeptin on gene expression of the follicle-stimulating hormone β subunit (fshβ) and lhβ, as well as possible effects of incubation time on the pituitary of Nile tilapia.
MATERIALS AND METHODS
Fish used in this experiment were juvenile Nile tilapia (n=400, body length: 8.1±0.4 cm, body weight: 10.2±1.4 g) that were hatched and raised at Sun Moon University (Asan, Chung Nam, Korea). To minimize the effects of endogenous sex steroids on kisspeptin’s action, juvenile tilapia at immature stages (GSI<0.25%) were used in the present study. All fish were raised in a filtered system, at 27±1°C, with a photoperiod of 14 hours light: 10 hours dark, and were given dry commercial feed (Woosung, Seoul, Korea) twice daily. Fish were anesthetized with benzocaine (50 ppm) under sterile conditions for pituitary extractions. Extracted pituitaries were placed in 96-multiwell plates with a culture medium (pH 7.4) composed of Leibovitz’s L15 medium 80% (v/v) (Gibco® BRL, Bethesda, MD, USA), fetal bovine serum (10%), penicillin (100 IU/mL), and streptomycin (100 μg/mL). To stabilize the tissue, the pituitaries were cultured in the dark at 27°C for 3 hours. All animal maintenance and experimental procedures were approved by the Institutional Animal Care and Use Committee of Sun Moon University.
A Kiss2 peptide (Kp-10, FNYNPLSLRF-NH2) was synthesized and purified using high-performance liquid chromatography according to the manufacturer’s procedures (Peptron, Daejeon, Korea). The purity of peptides used in study was >95%. The peptide was diluted in phosphate-buffered saline (PBS).
Pituitary culture experiments were conducted with different concentrations of Kp-10 and differing incubation times. The composition of culture medium were decided according to a previous report ( Jin et al., 2016). First, for concentration experiments, stabilized pituitaries were placed in culture medium (Leibovitz’s L15 medium 80% (v/v), fetal bovine serum 10%, penicillin 100 IU/mL, streptomycin 100 μg/mL, pH 7.4) with different concentrations of Kp-10 (0, 0.001, 0.01, 0.1, 1, or 10 μM) for 3 hours (n=5−9 per experimental group).
For incubation time experiments, stabilized pituitaries were placed into culture medium (Leibovitz’s L15 medium 80% (v/v), fetal bovine serum 10%, penicillin 100 IU/mL, streptomycin 100 μg/mL, pH 7.4) with either Kp-10 (0.1 μM), salmon luteinizing hormone releasing hormone (LHRH) (0.1 μM) (Sigma-Aldrich, St. Louis, MO, USA), or both Kp-10 and LHRH (Kp-10+LHRH) (0.1 μM) for 3, 6, 12, or 24 hours (n=5−9 per experimental group). The concentration used for incubation time experiments was 0.1 μM, selected because it was the concentration that affected the gene expression of GtH subunits in Kp-10 concentration experiments. As a control, stabilized pituitaries were cultured in the base medium in the absence of peptides. All experimental pituitary tissues were stored at −70°C prior to total RNA extraction.
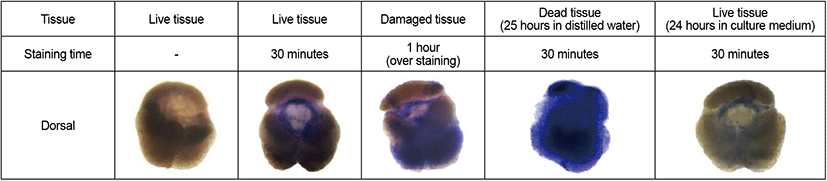
Trypan blue staining was used to verify the viability of pituitary function under the conditions used in culture experiments. Live pituitaries (control), dead pituitaries (immersion in distilled water), and pituitaries cultured for 24 hours in the experimental culture medium were stained with 0.4% trypan blue for 30 minutes (n=3 per experimental group). In addition, to identify any tissue damage from the trypan blue toxin, live pituitary tissue was stained with 0.4% trypan blue for an hour (n=3). All stained pituitaries were washed with PBS until unincorporated stain was removed, and were observed using a 3D optical microscope (EZ4, Leica, Germany).
Total RNA was extracted using TRI reagent® (Invitrogen, Waltham, Ma, USA). All reagents used in cDNA synthesis were purchased from Promega (Madison, WI, USA). Each total RNA (1 μg) sample was treated with DNase I (RQ1 RNase-free DNase) to remove genomic DNA and, then, reverse transcribed according to the manufacturer’s protocol using Oligo (dT)15 primer, 10 mM dNTP mix, M-MLV (Moloney murine leukaemia virus) reverse transcriptase and the buffer provided.
qRT-PCR was performed to quantify changes in the expression of fshβ and lhβ in the pituitary of Nile tilapia. Pituitaries were cultured with different concentrations of Kp-10 and were also cultured with 0.1 μM Kp-10, LHRH, and Kp-10+LHRH for various lengths of time. All primers used in the experiment were produced using Beacon Designer (Bio-Rad, Hercules, CA, USA) (Table 1). All qRT-PCR reactions were conducted using Topreal™ qPCR 2X PreMIX SYBR Green (Enzynomics, Daejeon, Korea) and analysed with the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad). Reaction conditions for qRT-PCR were as follows: Initial denaturation at 95°C for 15 minutes, followed by 45 amplification cycles of denaturation at 95°C for 15 seconds, annealing at 60°C for 15 seconds, and elongation at 72°C for 30 seconds. Following the reaction, the melting curve was analysed. GAPDH mRNA was used as the reference and CFX Manager™ (Bio-Rad) was used for relative quantification using the 2−ΔΔCt method. All samples were analysed a minimum of two times.
The expression levels of fshβ and lhβ (mean±SEM) in pituitary tissues obtained from culture experiments using different concentrations of Kp-10 were analysed using a one-way ANOVA and Duncan’s multiple range test (p<0.05). The expression of fshβ and lhβ (mean±SEM) obtained from culture experiments with various incubation times in media with Kp-10, LHRH, or Kp-10+LHRH were analysed using an independent t-test (p<0.05) on each experimental group and the control group (0 μM). All statistical analyses were performed using SPSS, version 18.0 (IBM, Armonk, NY, USA).
RESULTS
There were distinct differences between live, damaged, and dead pituitary tissues (Fig. 1). Pituitaries cultured in the medium for 24 hours were not stained with trypan blue, and neither were live pituitaries. However, damaged and dead tissues were heavily stained with trypan blue. These results indicate that there was no damage to pituitary tissues during pituitary culture experiments for 24 hours.
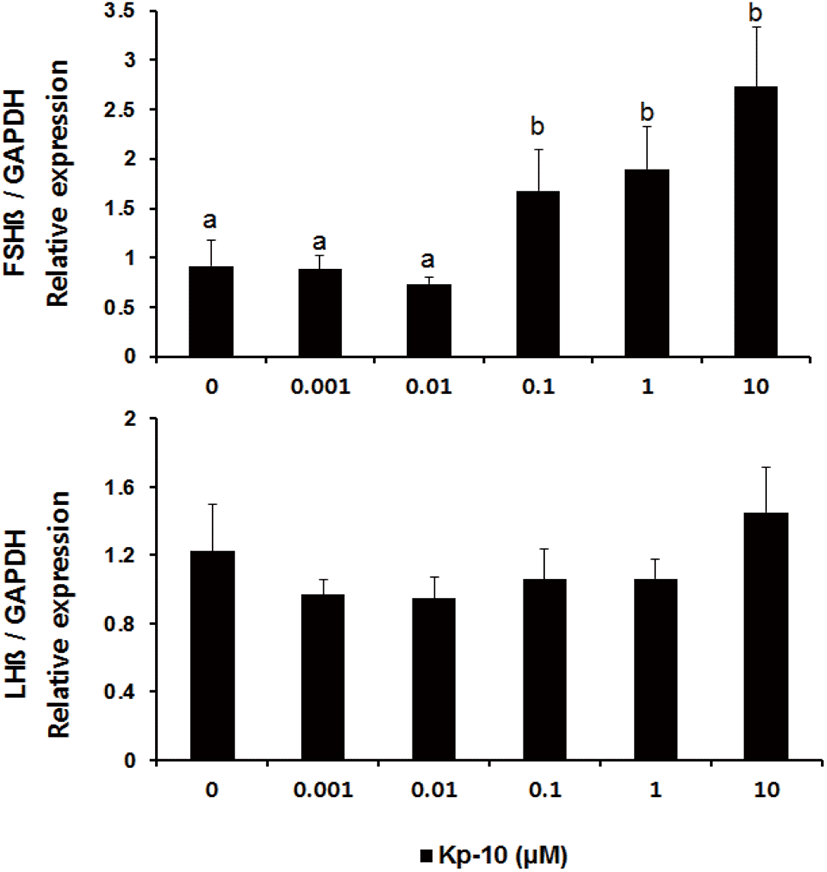
There was no difference in the expression of the fshβ gene (mean±SEM) in pituitary tissues in culture with Kp-10 at concentrations lower than 0.01 μM for 3 hours; however, levels of expression increased in response to concentrations higher than 0.1 μM (Fig. 2). In contrast, levels of lhβ mRNA did not exhibit significant differences in response to varying concentrations of Kp-10.
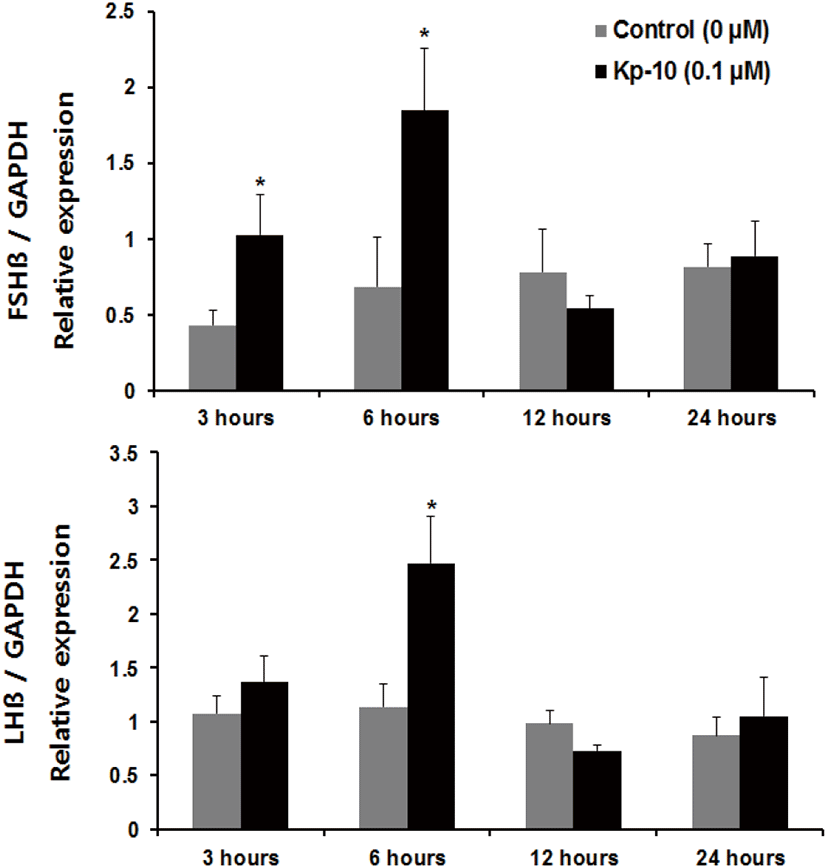
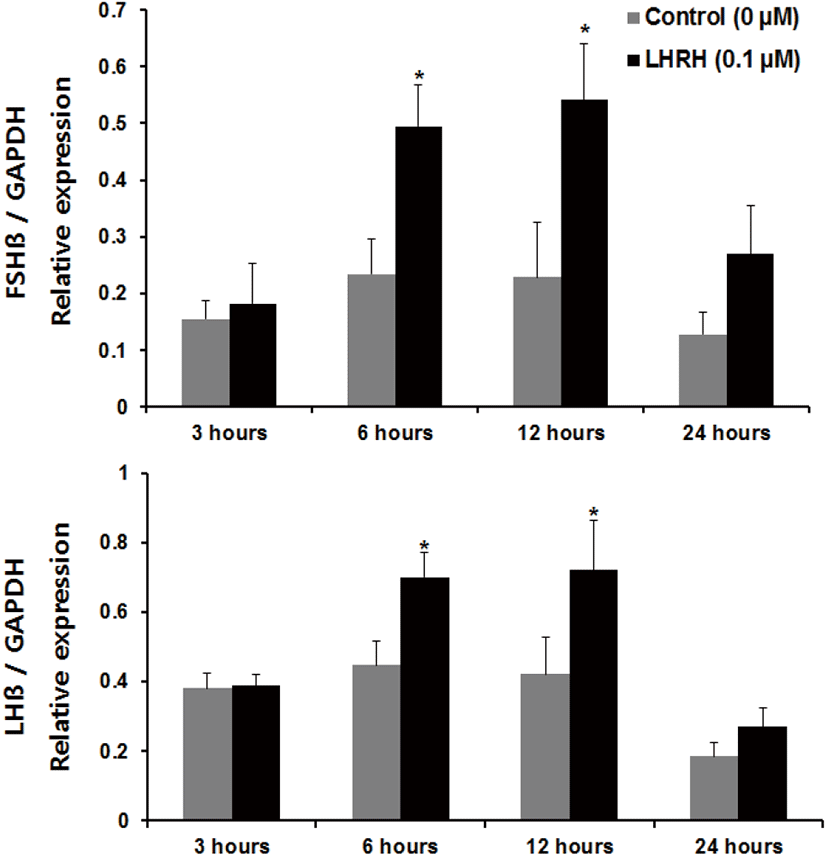
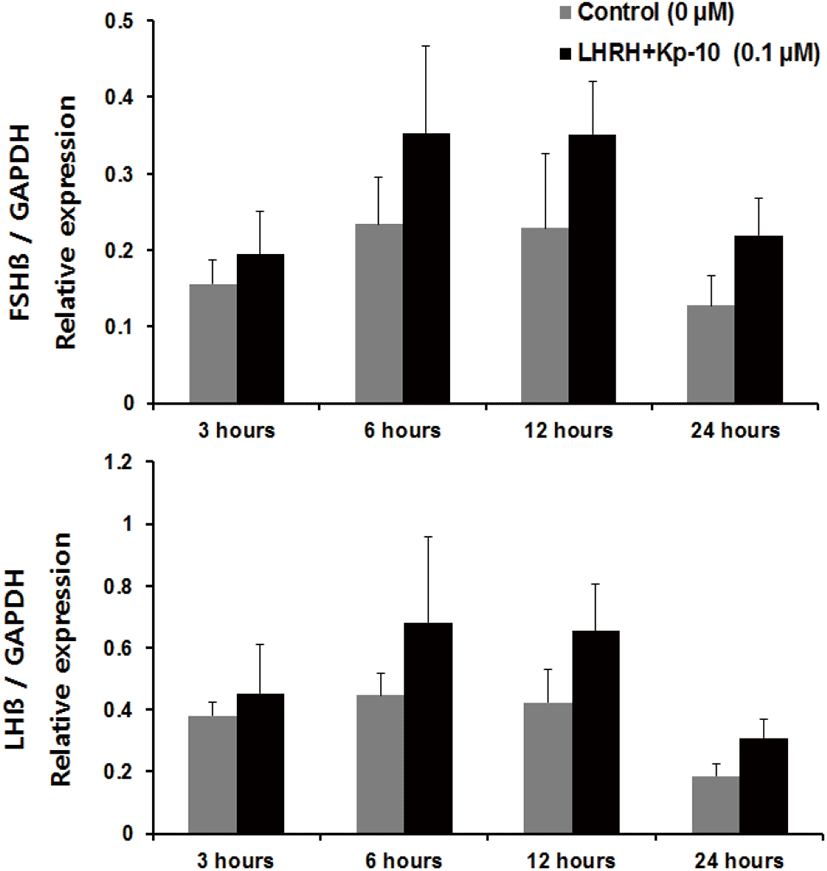
Regarding incubation times, we observed a significant increase in the expression of fshβ mRNA at 3 and 6 hours, and an increase in the expression of lhβ mRNA at 6 hours, compared with the control group of pituitaries cultured in the presence of Kp-10 (0.1 μM) (Fig. 3). However, there were no significant increases at 12 or 24 hours for either gene compared with that in the control group (Fig. 3). In pituitaries cultured in the presence of LHRH (0.1 μM), there were significant increases in the expression of fshβ and lhβ mRNA at 6 and 12 hours, compared with that reported for the control group, but no differences at 24 hours (Fig. 4). Lastly, there were no significant differences in the expression of fshβ and lhβ mRNA at any time when pituitaries were cultured in the presence of Kp-10+LHRH (0.1 μM), although the overall expression of these genes was higher than that of the control group (Fig. 5).
DISCUSSION
In this study, we investigated that kisspeptin could have a direct effect on the expression of GtH subunits in the Nile tilapia pituitary. The results show that treatment with Kp-10 increases the expression of the subunits of two gonadotropin genes in the pituitary of the Nile tilapia. However, the present study demonstrates that treatment with Kp-10 at different concentrations in the culture medium and for varying incubation times had different effects on the subunits of the gonadotropin genes. Incubating pituitary tissues of the Nile tilapia with at 0.1−10 μM Kp-10 for 3 hours increased fshβ mRNA levels, whereas no differences were detected in the levels of lhβ mRNA expression (Fig. 2). However, levels of lhβ mRNA expression increased after 6 hours of incubation at a constant concentration of 0.1 μM Kp-10 (Fig. 3). This finding could be the result of differences in the timing of lhβ expression, due to the delayed transcription of lhβ mRNA in response to kisspeptin. This phenomenon has also been observed in goldfish; kisspeptin treatment did not have any effects on lhβ mRNA expression after 180 minutes of incubation, but increased after 24 hours of incubation, suggesting the possibility of delayed gene transcription (Yang et al., 2010). However, an alternative explanation may involve differences in the effects of kisspeptin on the expression of the subunits of gonadotropin genes in the pituitary.
Increased expression of the two gonadotropin β-subunits relative to the control group was observed in response to treatment with Kp-10 after 6 hours of incubation, but no significant increase was seen at 12 or 24 hours for either gene relative to the control group. As shown by the viability experiment, this decrease in gene expression is not likely to be the result of damage to pituitary tissue. The kiss gene’s receptor is G protein-coupled receptor 54 (GPR 54) (Ohtaki et al., 2001). Studies of GPR54 receptor desensitization in female mice have reported that continuous kisspeptin treatment can reduce levels of LH and FSH initially elevated by kisspeptin exposure (Roa et al., 2008). Considering these facts, long-term treatment with kp-10 may lead to GPR-54 receptor desensitization in the pituitary gland of Nile tilapia. However, it is difficult to explain the decrease in gonadotropin β-subunits over time based on the results of this study alone. Thus, further studies are needed.
In vertebrates, GnRH is known to be an important regulator of GtH secretion in the pituitary. Treatment with GnRH in mammalian pituitary cells increased LH secretion to a greater extent than kisspeptin treatment (Gutiérrez-Pascual et al., 2007), and GnRH was a potent regulator of reproductive processes. Similarly, in the present study, the increased expression of gonadotropin β-subunits in the presence of LHRH was maintained for a longer period than that in tissues cultured in Kp-10-supplemented medium. In the present study, LHRH was used as a positive control, as GnRH is known to stimulate GtH secretion in vertebrates, including teleosts. Our findings suggest that GnRH is more effective at increasing the mRNA expression of the gonadotropin β-subunits in the pituitary of the Nile tilapia than kisspeptin. This difference might be explained by the fact that kisspeptin mainly acts on the hypothalamus; its action in the pituitary is only auxiliary.
In the present study, kisspeptin and LHRH effectively increased the mRNA expression of fshβ and lhβ. However, combined treatment with kisspeptin and LHRH peptides did not result in significant increases in the expression levels. This result could be explained as follows. First, when two substances with similar biological effects act simultaneously, they may offset each other, producing an antagonistic effect. Specifically, as LHRH and kisspeptin have the same effect of increasing GtH subunit expression in the pituitary gland, a compensating reaction may have occurred between the two molecules. Similarly, in in vivo experiments on yellow kingfish (Seriola lalandi), administration of Kiss1 alone increased GtH subunit levels, but administration of both Kiss1 and Kiss2 together did not lead to any such increase (Nocillado et al., 2013). Second, kisspeptin may have affected GtH subunit expression by reducing the sensitivity of GnRH secretion. In the known GtH secretion pathways, kisspeptin stimulates GnRH neurons in the hypothalamus, causing the secretion of GnRH, which in turn stimulates GtH secretion in the pituitary gland. However, in the present study, the combination of kisspeptin administration with continuous GnRH stimulation may have reduced the sensitivity of GnRH secretion, thus affecting GtH subunit expression as well. A study that used rhesus monkeys (Macaca mulatta) reported that long-term kisspeptin treatment decreased the sensitivity of GnRH secretion in the hypothalamus (Seminara et al., 2006). In addition, several studies on mammals have reported that continuous kisspeptin treatment downregulates hormones of the reproductive axis (Thompson et al., 2006; Ramaswamy et al., 2007). In fish case, in vivo experiments on striped bass (Morone saxatilis), GnRH showed a decreasing trend as the concentration of administered Kiss2 was increased (Zmora et al., 2012). Furthermore, in in vitro experiments in striped bass pituitary cells, levels of GnRH tended to decrease as the concentration of administered Kiss2 was increased (Zmora et al., 2015). These results suggest that the expression of kisspeptin and GnRH may have mutual effects. However, the results of the present study do not suggest an explanation for either antagonism or synergism between GnRH and kisspeptin in the Nile tilapia, and thus further research is needed.
To summarize, we investigated the direct regulatory function of kisspeptin on fshβ and lhβ in the pituitary of the Nile tilapia at different concentrations and incubation times. The results showed an increase in the expression of fshβ and lhβ in pituitaries cultured in Kp-10 (0.1 μM) for 6 hours and in LHRH (0.1 μM) after 6 and 12 hours of incubation. Pituitary tissues cultured in a mixed medium of LHRH and Kp-10 (0.1 μM) did not show significant increases in the expression of fshβ and lhβ. These results provide experimental evidence for the direct function of kisspeptin on the gonadotropins fshβ and lhβ in the Nile tilapia, excluding indirect effects via the hypothalamus. The findings suggest that there may be an additional reproductive pathway apart from the existing one linking the hypothalamus, pituitary, and gonads. It is also possible that the Kiss gene found in the pituitary might perform a function different from that of the Kiss gene found in the hypothalamus. However, our findings do not sufficiently illuminate the function of the Kiss gene in the pituitary. Yang et al. (2010) reported that kisspeptin in the pituitary of goldfish stimulates the secretion of prolactin and growth hormone, suggesting that it might be performing other functions than mere reproduction. Follow-up studies are needed to further clarify the function of the Kiss gene in the pituitary of Nile tilapia.