INTRODUCTION
It is evident that nonylphenol (NP), a commonly used industrial chemical, is an endocrine disrupting chemical (EDC) with estrogenic activity exerting adverse effects on health (Soto et al., 1991; Noorimotlagh et al., 2017; Rattan et al., 2018). In rodents studies, ample evidence has proven that NP could interfere with reproductive system (Nagao et al., 2001; Han et al., 2004; Gong et al., 2009; Mehranjani et al., 2009; Uguz et al., 2009; Aly et al., 2012; Lu et al., 2014). In addition, we also demonstrated that the subchronic low-dose NP exposure resulted in adverse effects on the reproductive parameters of male and female F1 mice, such as decreased weight of ovary and uterus in F0 animals, decreased rate of ovulation in female F1 offspring, increased weight of the testis, decreased weights of the epididymis, prostate and seminal vesicle in male F1 offspring (Cha et al., 2017; Kim et al., 2019). In the present study, we used the identical subchronic low-dose NP exposure model to clarify the potent pathohistological effect of NP exposure on the reproductive organs in F1 female mice.
MATERIALS AND METHODS
ICR mice were provided by DBL (Chungcheongbuk-do, Korea) and reared in Sangmyung University animal facility under photoperiods of 12 h light/dark with lights on at 7 AM and constant temperature of 21°C–23°C. Food and tap water were supplied ad libitum. The animal protocols were approved by the Animal Care and Use Committees at Sangmyung University (approval number R-1601-1). All the animals received humane care in accordance with the guides for animal experiments of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
After acclimation (2 weeks), breeding pairs of mice were divided into 2 groups; (1) Control (CTL), supplied tap water, and (2) NP-50, treated with low-dose NP (50 μg/L; Sigma-Aldrich, St. Louis, MO, USA) via drinking water. The parental (P) generation mice had pre-mating period for 2 weeks. After pre-mating period, male and female mice were placed in the breeding cage until a vaginal plug of female mice was observed. The animals were treated with NP from pre-mating period to weaning of offspring (F1). The F1 generation mice were weaned at postnatal day (PND) 21 and consistently treated with the NP until sacrifice at PND 30. The dates of vaginal opening (VO) were measured until PND 33. Animals were sacrificed, and sample collections were done between 18:00–19:00 pm. The tissues were fixed in paraformaldehyde (4%) for histological study.
Fixed tissues (ovaries and uteri) were dehydrated in graded concentrations of ethanol (70%, 80%, 90%, 95%, and 100%; Duksan, Korea) for 1 hour 30 minutes in each with gentle shaking and soaked in absolute ethanol overnight. The tissues were immersed in xylene (Samchun Chemical, Seoul, Korea) for 30 minutes, 3 times and in paraffin at 56°C for 30 minutes, 3 times. The tissues were embedded in paraffin and sectioned (Microm, Walldorf, Germany) at 5 μm. The samples were attached on microscope slides and the slides were stained with hematoxylin (Sigma-Aldrich) and eosin (Across, USA) for 5 minutes, respectively.
RESULTS
To find the effect of subchronic low-dose exposure to NP on the weight gain of F1 female mice, we measured and compared the body weights at weaning day (PND 21) and at sacrifice day (PND 30) (Table 1; n=9). The initial body weights of the NP-50 group animals were significantly lower than those of control group animals (p<0.001). However, the terminal body weights of the NP-50 group animals were not significantly different than those of control animals. Consequently, the increment rate of body weight in NP-50 group animals were significantly lower than those in control group animals (p<0.001).
| CON | NP-50 | |
|---|---|---|
| Initial body weight (g) | 10.6±0.2 | 9.0±0.2*** |
| Terminal body weight (g) | 23.2±0.4 | 23.2±0.3 |
| Increase rate of body weight (%) | 218±3 | 259±5*** |
Values are expressed as mean±SE (n=9).
NP, nonylphenol; PND, postnatal day; CON, control group provided with tap water only; NP-50, treated with nonylphenol at a dose of 50 μg/L.
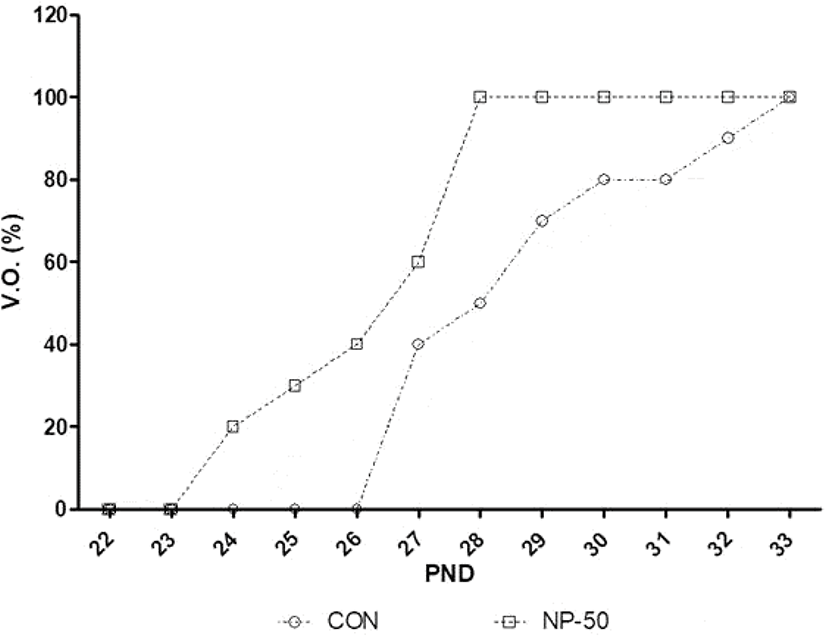
Fig. 1 indicates that advanced VO was occurred in NP-50 group animals compared to those in control animals. The average date of VO in control and NP-50 group is 28.9±0.89 and 26.5±0.52, respectively (p<0.05, n=9).
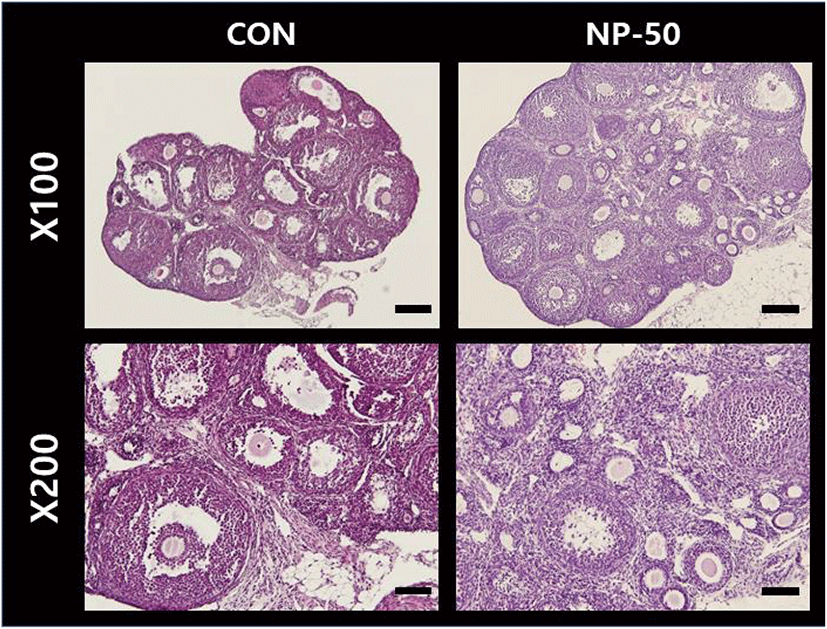
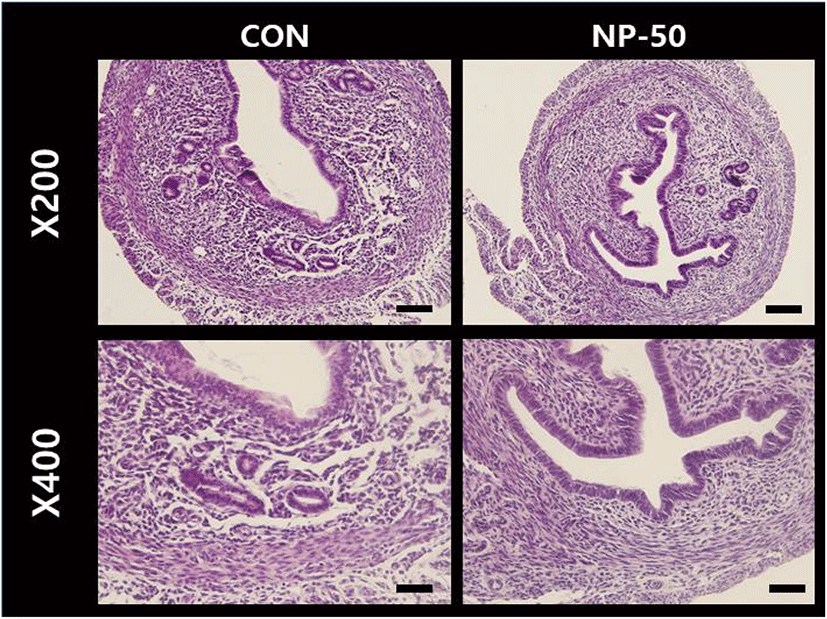
The overall size and shape of the NP-50 group ovaries were relatively bigger than control ovaries (Fig. 2). No corpus luteum (CL) was found in the control ovaries while several CL were present in NP-50 ovaries. Likewise, the primary and secondary follicles were more abundant in NP-50 ovaries (Table 2; n=4). The overall size and shape of the NP-50 group uteri were relatively smaller than those of control uteri (Fig. 3). Prominently thin layers of endometrium and myometrium, thick luminal epithelia, well-developed luminal structure and less number of gland were found in NP-50 group uteri (Table 2; n=4).
Values are expressed as mean±SE (n=4).
NP, nonylphenol; CON, control group provided with tap water only; NP-50, treated with nonylphenol at a dose of 50 μg/L.
DISCUSSION
The present study demonstrated that the subchronic low-dose NP exposure induced early beginning of puberty and pathohistological abnormalities in ovary and uterus of F1 mice. Previously, we demonstrated that the chronic lower-dose NP administration induced significant alterations such as weight of gonads and accessory organs and number of ovulated oocytes in F1 offspring (Cha et al., 2017). Interestingly, NP-induced changes were found in the absolute weight of the testis and epididymis but not in the relative weights of these organs (Kim et al., 2019). In the same study, however, histological examinations revealed that NP-treated F1 males exerted some extent of abnormalities; decreased seminiferous tubule diameters, reduced luminal area, and lower number of germ cells, and some sloughing morphologies in the tubules. Therefore, our previous and the present studies suggest that subchronic low-dose NP exposure could bring about reproductive toxicity without noticeable weight changes in gonad and/or accessory sex organs. We referred to this as ‘qualitative’ changes in reproductive tissues that could be derived from the epigenetic reprogramming by low-dose NP exposure which is frequently experienced in daily living (Kim et al., 2019).
NP can mimic the endogenous hormone 17β-estradiol, and it competes with the endogenous hormone for binding with the estrogen receptors (Soto et al., 1991). Although the concentration of NP in the environment is decreasing, it is still found at concentrations of 4.1 μg/L in river water and 1 mg/kg in sediments (Soares et al., 2008). Officially approved NOAELs (No Observed Adverse Effect Levels) and LOAELs (Lowest Observed Adverse Effect Levels) in rat for reproductive toxicity (based on decreases in epididymal sperm density or testicular sperm head counts, increases in estrous cycle length, and decreases in ovarian weights) range from 13 to 19 mg/kg-BW/day, and from 43 to 64 mg/kg-BW/day, respectively (EPA, 2009). The calculated oral NOAEL for repeated dose for humans was 15 mg/kg/day based on rat studies (Bontje et al., 2012). The dose employed in our previous and the present studies (18.5–19.8 ug/kg/day) was far lower than NOAEL in rat and human (Cha et al., 2017; Kim et al., 2019). Indeed, the average daily human intake of NP was calculated to be 7.5 ug/day in Germany and to be about 25.32 ug/day in Taiwan (Guenther et al., 2002; Lu et al., 2007). Therefore, we believe the dose used in our studies is much closer to the human daily intake level, and our NP treatment regimen is better to obtain practical information on the adverse effect of NP exposure to human (Kim et al., 2019).
In the present study we found that the initial body weights of the NP-50 group animals were significantly lower than those of control group animals, and the weight deficit were rapidly recovered when the terminal body weights were measured (Table 1). We hypothesized that 1) the low body weight of NP-50 group animals at weaning day could be caused by the adverse effect of NP exposure on the growth of F1 animals during intrauterine and/or lactational period, and 2) the fast catch-up with weight deficit in NP-50 group could be obesogenic effect of NP during prepubertal period. Concerning the weight gaining, there is an environmental obesogene hypothesis that proposes the exposure to endocrine disruptors during developmental “window” contributes to adipogenesis and the development of obesity (Hao et al., 2013). Further studies are needed to understand the opposite NP effects on body weight gain during these two periods. Fast weight gain by NP exposure during prepubertal period might be attributed to rapid accumulation of lipid within adipose, then this can be a driving force of early VO. Supporting this idea, previous studies demonstrated that body weight - or body fat - impacts on pubertal onset in female rats and monkeys (Glass et al., 1979; Boukouvalas et al., 2008; Terasawa et al., 2012).
In conclusion, the present study demonstrated that the subchronic low-dose NP exposure induced early beginning of puberty and pathohistological abnormalities in ovary and uterus of F1 mice. Further studies are needed to achieve a better understanding on the action mechanism of NP in pubertal onset and to find a way to avoid a hazardous situation provoked by NP exposure.

