INTRODUCTION
Di(2-ethylhexyl) phthalate (bis(2-ethylhexyl) phthalate, also known as diethylhexyl phthalate, Di-(2-ethylhexyl) phthalate (DEHP), dioctyl phthalate, DOP, Octyl phthalate; di-sec octyl phthalate) is the lead substance of phthalate esters, because DEHP has been widely used as a plasticizer in flexible vinyl products and largely produced among them. It is ubiquitously detected from environmental samples and organisms are easily exposed to it (Ito et al., 2019). In human, about half of DEHP is excreted as mono(2-ethylhexyl) phthalate (MEHP) (7.34%), 5-OH-MEHP (24.7%), and 5-oxo-MEHP (14.9%) within 2 days and the estimated serum elimination half-life of these metabolites is less than 2 h (Koch et al., 2004). Generally, the effects of phthalates are due to the monoester and metabolites derived therefrom. By the adverse effects of DEHP, in the United States and Canada, it is no longer used to manufacture for children’s products intended for mouthing. In the other countries also have been restriction of their usage through law. However, by its high product volume and common use, DEHP leach into the environment over time.
Phthalates were classified as endocrine disrupting chemicals (EDCs) in 2002 and DEHP has antiandrogen and estrogenic activities (Chen et al., 2014). By the in utero exposing of DEHP (100– 900 mg/kg/d from gestational d14–19 in rat), a reduced androgen production is induced and this is coincident with reduced mineralocorticoid receptor (MR) expression in Leydig cells with epigenic change in MR gene promoter at postnatal day 60 (Martinez-Arguelles et al., 2009). In estrogen receptor 1 (ER1) cell line, it shows a high agonistic and antagonistic activity, but a low agonist activity and a high antagonist activity in MVV-Luc cell line (Simon et al., 2016). It also suggested that DEHP induces the transactivation of ER (Chen et al., 2014). On the other hand, the risks of DEHP in carcinogenesis and child health has been focused with peroxisome proliferator-activated receptors (PPARs), because the metabolites of DEHP work as ligand of PPARs (Maloney & Waxman, 1999). It is classified as a potential carcinogen by the International Agency for Research on Cancer and the European.
Initial reproductive and developmental toxicity studies on phthalates did in pregnant female rodent and fetus. It showed various toxicity in implantation, reabsorptions, decreased body weight, and increased malformations in dose and developmental stage dependent manners (Tyl et al., 1988; Cheon, 2020). The well examined target organs of DEHP include ovary (Komar et al., 2001), testis (Li et al., 2014), endometrium, placenta, liver (Lovekamp-Swan & Davis, 2003), placenta (Martínez-Razo et al., 2021), renal, lung, immune system, brain development, and heart tissue (Martínez-Razo et al., 2021).
The absorption and metabolism of DEHP vary in vivo female by various factors such as species, physiological status. DEHP in CD-1 mice and Wistar rat is rapidly absorbed and reached to peak plasma levels with linearity with the absorption and a rapid increase is noted in mice but not in rats. The plasma half-life times ranged between 7.1 and 10.3 h at the low dose and between 5.5 and 13.5 h at the high dose. MEHP is dominant in blood both at the low and the high dose level. MEHP metabolites in blood are detected in rat but not in mouse. Repeated DEHP administration modify the metabolic pathway and decrease of DEPH excretion and increase of MEHP excretion in pregnant mice (Lorz et al., 2012). In marmosets, the organ/plasma ratio is generally 0.2 to 0.4 and does not alter the distribution by pretreatment for 65 weeks (Zeiger et al., 1985).
Even though the usage of DEHP in industry is strictly regulated and the environment is tried to control, huge amount of industrial products had been around for a long time already. Therefore, it could be chronically exposed with low concentration through air, water, soil, and food (United States Environmental Protection Agency, 2013). Recently, there are many scientists suggest that low dose phthalate impacts on health of animals such as fertility and steroidogenesis (Bloom et al., 2015) and become a big issue in this field (Vandenberg et al., 2019). As the previous results, the possibility of chronic exposures to low-dose DEHP is an effect as EDC in the uterus without effect on F1 fertility (Cha et al., 2017; Cheon, 2020). Chronic low dose exposure of DEHP caused increase the uterine wet weight, endometrial thickness, and the number of glands (Cheon, 2020). Besides, it caused decreased expression of estrogen receptors in uterus. From such results, it is suspected that the adrenal gland can be a target organ of low level of DEHP.
DEHP induces various effects on various cellular responses including modulation of transcriptome such as steroid hormone receptors and paracrine factors. It is suggested that DEHP cause of cancer, reproductive, developmental, and immune toxicities, and endocrine disruption effects. Its effects have been studied on reproductive organs, kidney, lung, immune system, and nervous system. Although the adrenal gland is a suspected target endocrine organ of DEHP and important for development, only limited information is available about the effect of DEHP. In this study, the histological changes and the expression profiles of steroidogenic genes were evaluated in chromic low dose DEHP exposed adult female mice.
MATERIALS AND METHODS
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health. CD-1 (ICR) mice were maintained under standard condition at the animal house of Sungshin University. Circadian rhythm was kept under the 14L:10D schedule with light-on at 06:00 and clean room system. Animals were fed food and drink as mentioned in Cha et al. (2017). In brief, estrogen-free rodent diet (2018 Teklad global 18% protein rodent diets; ENVIGA, Madison, WI, USA) and water in glass bottles with stainless steel sipper tubes were offered ad libitum.
Administration of DEHP (Catalog no. 36735, Sigma Aldrich, St. Louis, MO, USA) was followed OECD Guidelines for the Testing of Chemicals – Test Guideline No. 443. Ten to twelve-week-old animals were given DEHP at a dose of 133 or 1,330 μg/L (DEHP133 and DEHP1330, respectively) dissolved in drinking water for 10 weeks, considering that a mouse drinks approximately 4–7 mL of water daily. Control group was given water without test substances. Estrous cycle was checked by vaginal smearing daily for first 2 weeks, then each normal cycling female was chosen and bred with a fertile male for 2 weeks (n=10/each group). Copulatory plug was checked daily. After 6 weeks, animals were used for examination. The animal body weight was measured and then sacrificed. The adrenal glands were dissected and measured wet weight of them.
After measuring the weight, they were fixed with 4% buffered paraformaldehyde in PBS and routinely embedded in paraffin. The paraffin-embedded adrenal glands were serial sectioned (coronal section) at 4 μm and mounted on glass slides. Every tenth sections of an adrenal gland were chosen, deparaffinized with xylene, and hydrated with alcohol series. And they were stained by Hematoxylin–Eosin. To evaluate the possible changes of extracellular matrix (ECM), Masson trichrome staining was used. The stained tissues were observed under the light microscope (Nikon, Japan). For image analysis the freeware ImageJ (National Institutes of Health software) v1.33 and the Color Histogram plug-in, both downloaded from the NIH website (http://rsb.info.nih.gov/ij) were used as mentioned in previous report (Kim et al., 2015).
Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in H2O for 30 min. CYP11B1, CYP17A1, and DAX1 was localized according to the Vectastain ABC kit method (Vector Laboratories, Burlingame, CA, USA). Briefly, tissues were incubated with 1% normal blocking serum in PBS for 20 min and then incubated with CYP11B1 antibody (dilution 1:500, polyclonal antibody, Cat No: MBS2026360, MyBioSource com), CYP17A1 antibody (dilution 1:100, monoclonal antibody, Cat No: sc-374244, Santa Cruz Biotechnology), and NR0B1(DAX1, dilution 1:100, polyclonal antibody, MyBioSource com). After washed in PBS containing 0.1% triton X-100 (PBST) and PBS, tissues were incubated with anti-mouse IgG (Vector Laboratories). Tissues were washed and incubated with avidin-biotin-complex reagent containing horseradish peroxidase for 30 min. Tissues were washed and color development was achieved using DAB substrate. The tissues were counterstained with hematoxylin.
These studies were done with repeated measurements (10 animals/group). The data were presented as mean ±SED and analyzed using SAS. One-way ANOVA was used to evaluate statistical difference, followed by t-test was performed for comparisons of two means. p-values<0.05 were considered significant.
RESULTS
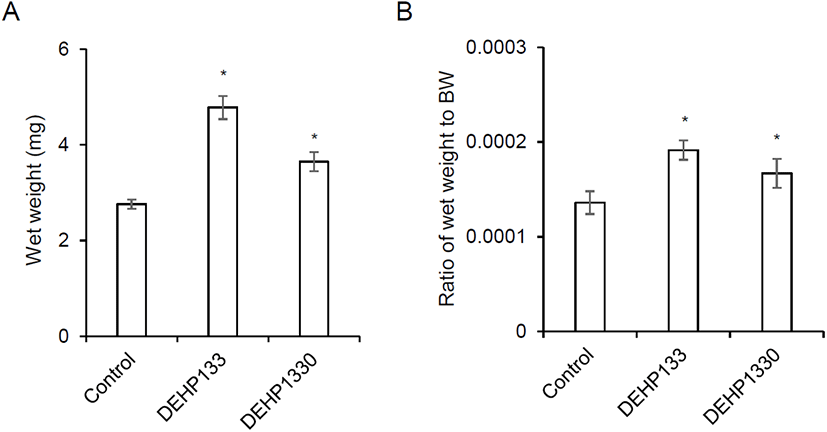
After drinking for 10 weeks, the mice were sacrificed and measure the wet weight as mentioned in Materials and Methods. The wet weight of adrenal glands was significantly increased in both 133 μg/L (p<0.05) and 1,330 μg/L (p<0.05) groups (Fig. 1A). The relative weight also significantly heavy in both (p<0.05) groups (0.0001, 0.0002, and 0.0002 in control, DEHP133, and DEHP1330, respectively) (Fig. 1B).
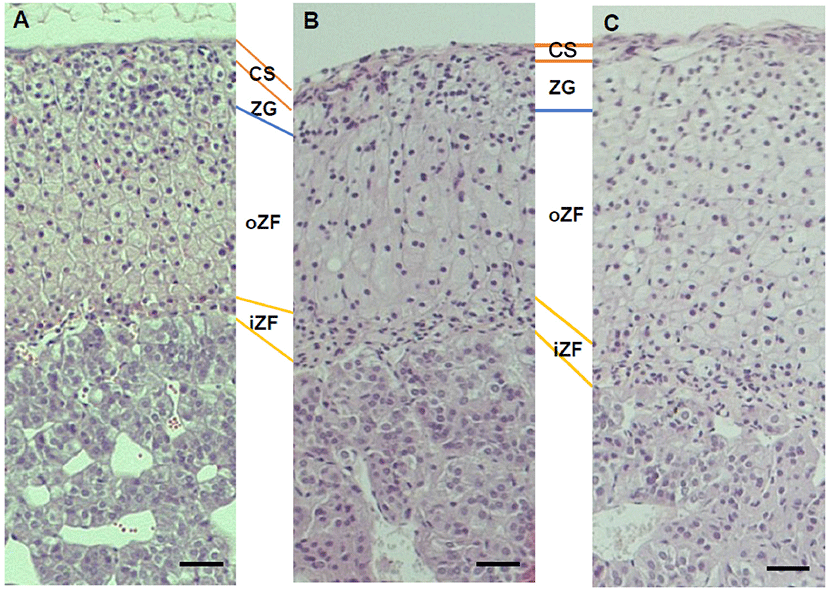
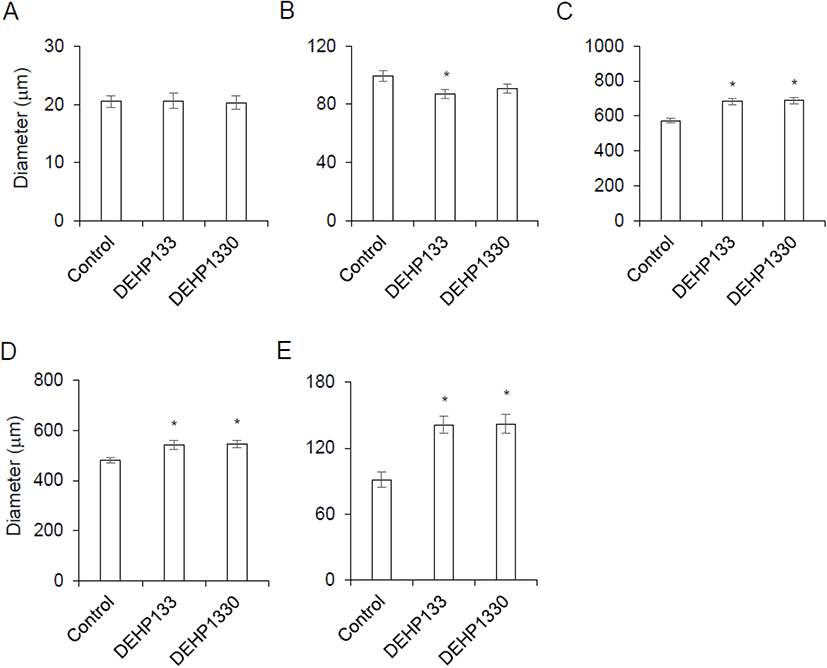
There was no histological change in cell shapes or grouping (Fig. 2) but the diameter of zona was not same between groups. The thickness of capsule was not different between groups (Fig. 3A). The diameter of zona glomerulosa (ZG) was decreased by DEHP and there was significantly difference (p<0.05) in DEHP133 group (Fig. 3B). However, the diameter of zona fasciculata (ZF) was increased by DEHP in both DEHP133 and DEHP1330 (Fig. 3C). The ZF can be distinguished likes outer ZF (oZF) and inner ZF (iZF). Comparing the diameter of oZF and iZF, it was increased significantly (p<0.05) in DEHP groups. the case of ZR, the diameters were significantly increased in both DEHP133 and DEHP 1330 groups in the concentration dependent manners (Fig. 3D, 3E).
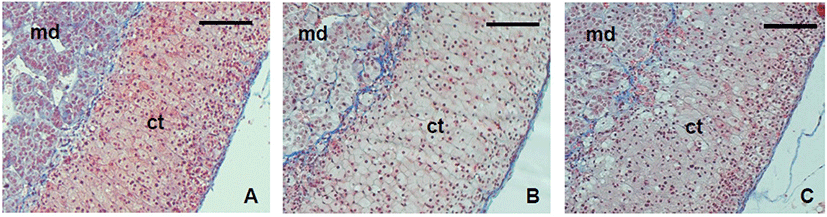
For the next step, the stability of the ECM was examined with the trichrome staining tool. The patterns of ECM deposit were not different between groups (Fig. 4). In the adrenal gland, collagen was strongly deposited in cortex and the border of medulla and cortex. It also localized between the cords.
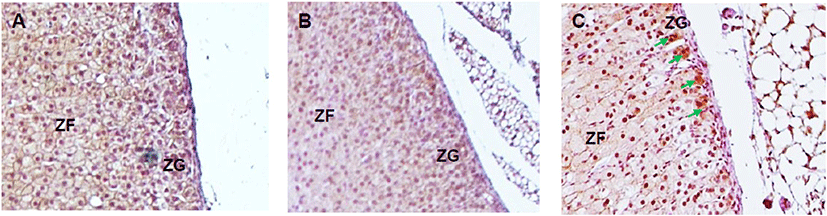
The expression of steroidogenic enzymes was evaluated with IHC. CYP17A1 is not expressed at adrenal cortex in laboratory mice in physiological status. Its expression was not detected in control and DEPH133 groups (Fig. 5A, 5B). In DEHP1330 group, the CYP17A1 specific staining was detected in ZG cells (Fig. 5C).
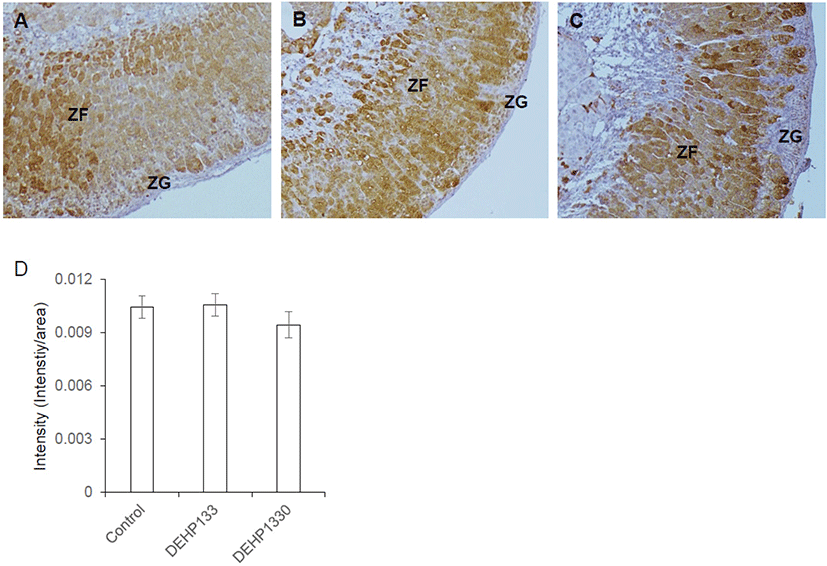
Cytochrome P45011B1 (11β-hydroxylase) respond for production of cortisol/corticosterone in adrenal cortex. It was predominantly localized in oZF and lesser in iZF. The staining intensity of it was not different between groups (Fig. 6A–6D). It means that the chronic low-dose DEHP administration did not affect on the expression of CYP11A1 in maternal mice.
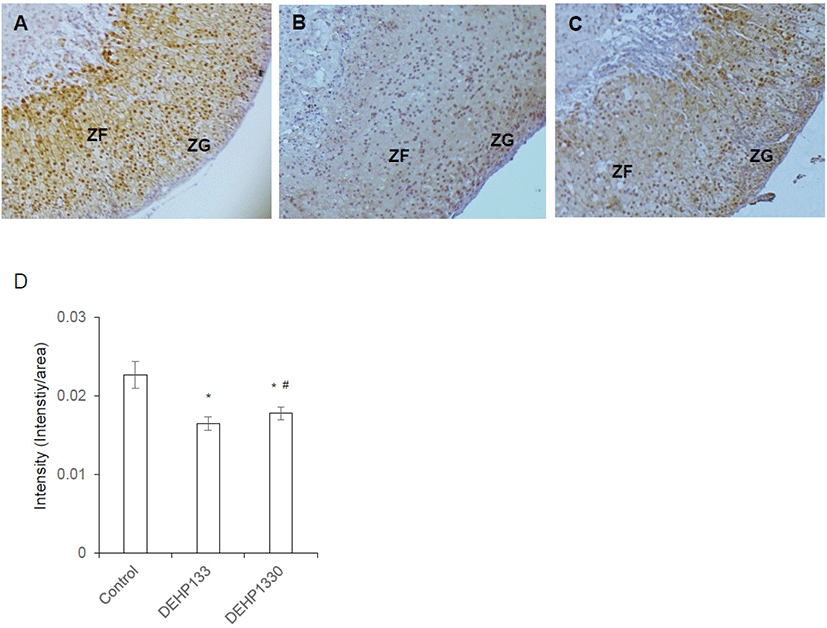
DAX1 is a nuclear receptor and has function as a regulator of steroidogenic enzyme induction and differentiation of adrenocortical cell. Its intensity was significantly decreased (p<0.05) in both DEHP133 and DEHP1330. The inhibitory effect of DEHP was stronger in DEHP133 group than DEHP1330 group (Fig. 7A–7D). In control mice, DAX1 was localized in nucleus of ZG and ZFs (Fig. 7A). In DEHP133 group, DAX1 was localized more strongly in the nucleus of ZG compared to ZFs (Fig. 7B). The nuclear localization of DAX1 in DEHP1330 group was similar with control group and localized in ZG and ZFs but weaker than that of the control (Fig. 7C).
DISCUSSION
OECD test guideline 443 provides a detailed description of the operational conduct of an extended one generation reproductive toxicity study and may be extended to include an F2 generation. In this study, the maternal female drunk for 10 weeks containing DEHP were evaluated. The adverse effects of DEHP in reproductive organs and development has been evaluated, and revealed the involvement of PPAR-dependent pathways in DEPH toxic effects. DEHP acts on the liver lipid metabolism and increases enzyme activities involved in mitochondrial and peroxisomal fatty acid β- and ω-oxidation through PPARs in species-specific manners. Low dose DEHP (1 mg/kg/day) exposing at uterus led DNA methylation changes at doses below those that affect aldosterone biosynthesis with decreased PPARα expression (Martinez-Arguelles & Papadopoulos, 2015). On the other hand, during adult exposing of DEHP effects on steroidogenesis pathway of various target organs (Ahmad et al., 2022). CYP11B1 is the classical 11β-hydroxylase which converts 11-deoxycortisol to cortisol and deoxycorticosterone to corticosterone. Cyp11b1 null cause of glucocorticoid deficiency, mineralocorticoid excess, and congenital adrenal hyperplasia (Zhang et al., 2020). Glucocorticoid secretion relies on the CYP11B1 and production is mainly under the control of the hypothalamo-pituitary-adrenal axis and ACTH/cAMP/PKA signaling (Dumontet & Martinez, 2021). An orphan nuclear receptor, DAX1 (NR0B1) is important in function of the adrenal gland. It is localized in subcapsular area and its expression is activated by glucocorticoids and inhibited by ACTH (Gummow et al., 2006; Kim et al., 2008). One of the roles of Dax1 is the maintenance of subcapsular adrenocortical progenitor cells (Scheys et al., 2011) and the hormonally induced changes in SF-1/DAX1 ratio cause the fine tune ACTH responsiveness of ZF cells (Ragazzon et al., 2006).
The adrenal cortex is a unique morphological and functional zonation consisted with three cell types. These cells are developed from the mesenchymal tissue adjacent to the coelomic epithelium near the urogenital ridge and an additional cell type originated from the mesonephros and the region of Bowman’s capsule (McCabe et al., 2001). The adrenal cortex is clearly subdivided into ZG, ZF, and ZR in human and some primates by cellular arrangements. ZR is considered unique to humans and certain primates (Turcu et al. 2018). In the case of the adult female Prkar1a AdKO, adrenal cortex can be classified as ZG, ZF, and ZR-like (Dumontet & Martinez, 2021). However, in adult and parous female and male laboratory mice, it can be classified as two (ZG, ZF) or three (ZG, oZF, and iZF) (Dumontet & Martinez, 2021).
The ZG is the source of cells for the inner two zones, fasiculata and reticularis, of the cortex. Mitotane can induce the destruction of glomerulosa cells and cause the losing of fasiculata and reticularis (Lyraki & Schedl, 2021). On the other hand, by the feedback control, prolonged high level of glucocorticoids causes atrophy of the hypothalamic-pituitary unit and the zonae fasiculata and reticularis. In the histological characters in chronic low-dose DEHP groups with H-E and trichrome staining, showed normal cell shapes and arrangement in the zonas with thickened ZFs, but not change in ECM localizing patterns. DEHP has a function as a modulator of cell proliferation and differentiation in various cells such as T-47D cell, endometrial stromal cell, thyroid cells, etc (Richardson et al., 2018; Crobeddu et al., 2019; Cheon, 2020). It is may cause of the increase of wet weight of adrenal gland. Though the molecular level studies are needed, it is suspected that chronic low-dose DEHP administration could be the cause of the stimulated differentiation of ZG cells to ZF cells.
Adrenal cortex is a steroidogenic tissue but has differences in the expression of P450 genes from other steroidogenic organs such as testis and ovary. The cytochrome P450 (CYP) genes Cyp51, Cyp11a1, Cyp17a1, Cyp11b1, Cyp11b2, and Cyp21a1 participate in the adrenal production of corticosteroids, glucocorticoids, mineralocorticoids, and adrenal androgen. These steroids are synthesized in zona-specific manners because steroidogenic enzymes are zona-specific expression. Aldosterone (a mineralocorticoid) is secreted by the ZG. Glucocorticoids, cortisol, corticosterone, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), androgens and estrogens are synthesized in the ZF and ZR. The expression of these steroids is under the influence of ACTH (Niakan & McCabe, 2005). In the case of mouse, Cyp17a1 expression does not occur in the adrenal after embryonic development and results in the production of corticosterone and the absence of DHEA and DHEAS (Martinez-Arguelles & Papadopoulos, 2015) in epigenic modification. Cyp17a1 is express in adrenal gland of Crem knockout mice through epigenetic regulation (Košir et al., 2012). Interestingly, in DEHP1330 group, the CYP17A1 specific staining was detected. It is suggested that the possibility of the epigenic effect of chronic low-dose DEHP in adrenal cortex.
Previously a few groups including Martinez-Arguelles & Papadopoulos (2015) suggested that adrenal cortex is a target of DEHP as in Leydig cells of testis. Exposing to 100 mg/kg/day DEHP appears to be the threshold for adult endocrine disruption following an in utero exposure. DEHP effects on the adrenal gland as EDC and exerts long-term effects. DEHP decreases the expression of steroidogenic acute regulatory protein (StAR) expression in both mouse and human. It also decreases the levels of 17α-hydroxylase (CYP17A1) and cytochrome P450 (Martinez-Arguelles et al., 2009; Kariyazono et al., 2015). Fetal exposure resulted in altered ZG development in a dose- and time-specific manner (Martinez-Arguelles et al., 2011). Interestingly in maternal group, the expression level of CYP11A1 was not affected by chronic low-dose DEHP administration. However, DAX1 expression was dramatically decreased. Put together, it is suggested that adrenal cortex can be a target of chronic low-dose DEHP.
In summary, the cell shapes and arrangement in zonas were not changed by the chronic low-dose DEHP administration but the thickness of ZF was increased. The expression levels of CYP11B1 was not different between groups but not in DAX1. Its expression was decreased by DEHP. In addition, the CYP17A1 specific signaling was detected in ZG of DEHP1330 group. Previously we showed that the administration of DEHP with the strategy of OECD test guideline 443 did not cause of decrease of litter size (Cha et al., 2017). Put together, these results suggest that chronic low-dose DEHP exposing may cause modified structure and function of adrenal cortex to compensation for DEHP disturbance as EDC.

