INTRODUCTION
In teleost, the developmental patterns leading to the expression of gonadal sex are controlled by genetic, environmental and social factors. Different patterns of gonadal development have been described, including gonochoristic and hermaphroditic species (Nagahama et al., 1998; Devlin & Nagahama, 2002; Guerrero-Estevez & Moreno-Mendoza, 2010). Gonochoristic species may be differentiated or undifferentiated. In the differentiated gonochoristic species, early gonad development proceeds from an indifferent gonad directly to ovary or testis (Piferrer & Donaldson, 1989; Lee et al., 1996); in the undifferentiated species, all individuals develop an initial gonad similar to an ovary, which subsequently differentiated into a testis in approximately half of the population, and into a proper ovary in the other half (Guerrero-Estevez & Moreno-Mendoza, 2010). Some gonochoristic species go through a period where all gonads are initially intersexual prior to differentiation into either testis or ovary (Sadovy & Colin, 1995; Asoh & Shapiro, 1997).
The gobies, mostly small are one of the largest families in the Perciformes. They are distributed worldwide, in marine, estuarine and freshwater habitats (Nelson, 1984). Many of the gobiid fish have one or two years total lifespan. Recently, the external environmental changes and contaminants’ influencing the gobiid fish have been studied intensively (Baek et al., 2004, 2007; Mochida et al., 2004; Ito et al., 2007; Robinson et al., 2007; Saaristo et al., 2009). However, little is known about early gonad development in fishes in general, and gobies in particular. Gobiid fish exhibit a variety of sexual patterns including gonochorism, simultaneous hermaphroditism and protogyny (Cole, 1990).
The chameleon goby, Tridentiger trigonocephalus, is in the genus Tridentiger of the family Gobiidae. Their habitats extend from China, Japan, Russia, to the northwest region of the USA, and include the mudflats in the southern and western coastal waters, brackish and freshwater in South Korea (Chung, 1977). In the previous study, we investigated the reproductive cycle of chameleon goby (Hwang & Baek, 2013). The aim of this study was to describe early gonadal development and determine the timing of gonadal differentiation using the histological method.
MATERIALS AND METHODS
The experimental fish were collected in the eelgrass bed around Dongdae Bay, Namhae, Gyeongsangnamdo, South Korea using a scoop net. Male and female were maintained under a photoperiod of 14h-light and 10h-dark at 21±0.5°C in 90 L indoor aquarium system. Polyvinyl-coated (PVC) pipes (5 cm in diameter and 12 cm long) were used as spawning substrates. Substrates containing eggs were collected from the brood stock aquaria and were placed in a 30 L glass aquaria. As soon as the hatching completes, substrates were removed from aquaria. Larvae were fed with rotifer, Brachionus rotundiformis alone for 15 days after hatching (DAH), rotifer and brine shrimp, Artemia nauplii for the next 5 days, and brine shrimp for the next 10 days. Fish older than 30 DAH were fed with artificial feed until the end of the experiment.
Larvae samples (from 1 to 100 DAH) were collected for the gonadal histological study. Its samples (5 fish) were collected every 3 days between day 1 to 15, every 5 days between day 15 to 70, and every 10 days between day 70 to 100.
For the histological observation, larvae and juveniles were fixed in Bouin’s solution for 24 hours and then embedded in paraffin. The paraffin embedded specimens were sectioned in 5 to 6 μm thick sections. The sections were stained with Mayer’s hematoxylin-eosin, and observed under a light microscope (BX 50, Olympus, Japan).
RESULTS
During experimental period (from 3 to 100 DAH), total length of chameleon goby larvae and juveniles ranged from 2.6 to 38.8 mm respectively. Growth curve in total length related to DAH was estimated by the Gompertz growth curve. The growth curve of chameleon goby larvae and juvenile could be expressed as (Fig. 1):
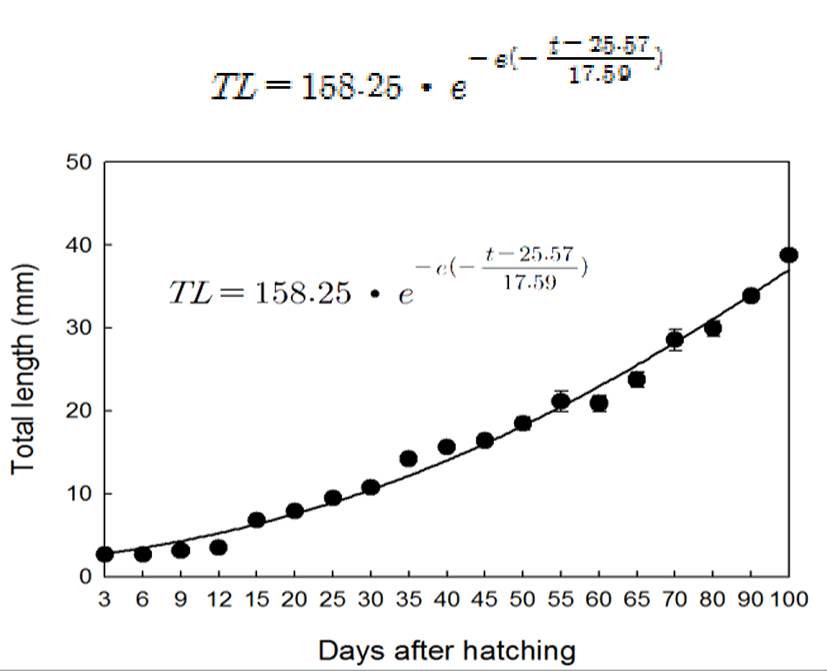
The primordial germ cell (PGC) was observed in mesentery between mesonephric duct and gut at 15 DAH (total length, TL: 6.8±0.2 mm). At this stage, PGCs with large nuclei which were intensively stained by hematoxylin were located in the germinal epithelium line adjacent hindgut. The diameter of PGCs ranged from 10 to 12 μm (Fig. 2-A, B). At 20 DAH (TL: 7.9±0.1 mm), the primordial gonad with a few somatic cell developed and began to protrude into the peritoneal cavity between mesonephric duct and gut (Fig. 2-C, D). At the 30 DAH (TL: 10.7±0.3 mm), the germ cells were surrounded by somatic cells and distinguished from the somatic cells by their larger size. Nuclei of the germ cells were stained by hematoxylin more intensively than cytoplasm (Fig. 2-E). At 45 DAH (TL: 15.6±0.3 mm), the primordial gonads were elongated and the number of germ cells and somatic cells increased by mitosis of the cells (Fig. 2-F).
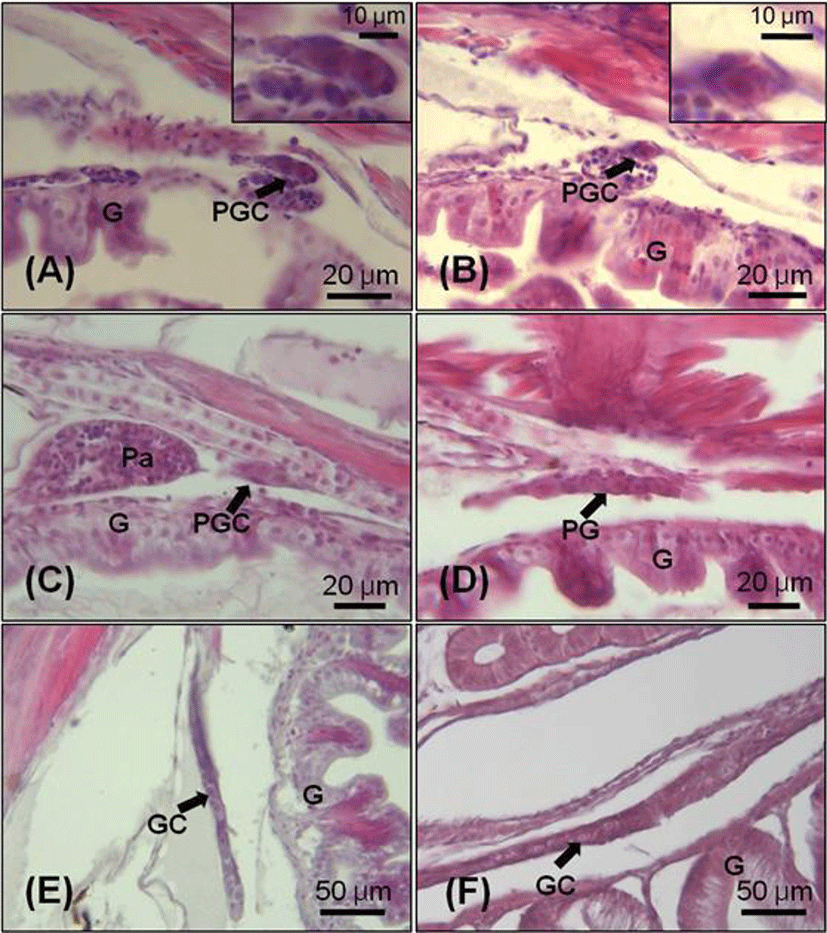
Initial ovarian differentiation was identified by the presence of ovarian cavity and transformation of PGCs to meiotic oocytes. The oogonia were first observed in the gonads at 55 DAH (TL: 21.1±1.3 mm), which indicate that oogenesis already initiated within the gonad. The oogonia at this stage were increased in number and diameter range from 20.0 to 35 μm (Fig. 3-A). In 70 days juvenile (TL: 28.6±1.3 mm), the ovary started to fill with perinucleolus oocytes (Fig. 3-B). At the 100 DAH (TL: 38.8±0.5 mm), the gonad was rapidly increased in size compared with those at 70 DAH (Fig. 3-C, D).
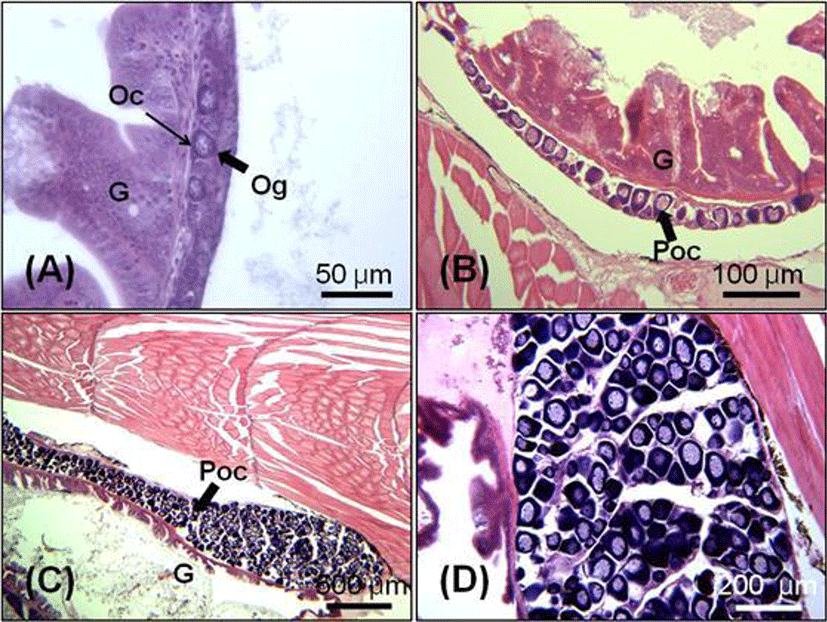
At 65 DAH (TL: 23.7±0.9 mm), the testicular differentiation was identified by the presence of spermatogonial cells (Fig. 4-A). In 70 days juvenile (TL: 28.6±1.3 mm), the testis became bigger and the number of spermatogonia started to increase (Fig 4-B). Seminiferous tubules of testis were filled with numerous spermatogonia in 90 days juvenile (TL: 33.8±0.5 mm, Fig. 4-C, D).
DISCUSSIONS
Most teleost species are gonochoristic, i.e., individuals develop only as males or females and remain go through-out their life span (Yamamoto, 1969). Sex differentiation has been shown to begin first in the gonads of females than in males in the majority of the gonochoristic teleosts examined to date. However, the initial pathway of gonadal development may vary. Some species may develop gonads which are initially hermaphroditic, but later develop as ovaries or testicles (Devlin & Nagahama, 2002).
Results from this study show differentiation of ovarian tissue has been observed 55 days post hatching, whereas differentiation of testicular tissue occurs about 10–15 days later; germ cells mitosis in females and males were first observed at 55 and 65–70 days after hatching, respectively. Testicular development usually occurs later than ovarian differentiation, some weeks or months after the onset of gonad development in females of some species (Guraya, 1994; Nakamura et al., 1998). In some species spermatogenesis begins long after hatching, in O. mossambicus and O. niloticus, spermatogenesis starts 50–70 days after hatching (Nakamura & Nagahama, 1989), and in medaka, ~50 days after hatching (Matsuda, 2005). In the medaka, the female germ cells become differentiated much earlier than do male germ cells (Saito et al., 2007).
The processes of sexual differentiation in fish are highly plastic. Several species among the gobiids are protogynus hermaphrodites, such as the bluebanded goby (Lythrypnus dalli). This is predominantly a protogynus fish (Cole, 1990; Reavis & Grober, 1999) but is capable of bi-directional sex change (Reavis & Grober, 1999; Kroon et al., 2003; Rodgers et al., 2007).
Based on histological observation, the chameleon goby exhibit undifferentiated gonochorism where the undifferentiated gonad develops an initial gonad similar to an ovary, which subsequently differentiated into a testis, and this species can be classified as an undifferentiated gonochorist. However, at this time, histological evidence does not reveal distinction of female or male gonadal cell types.

