INTRODUCTION
Studies of early stages in the life cycle of fish are performed to obtain information such as morphological and physiological characteristics of eggs, as well as species-specific characteristics and developmental traits that can be observed during embryogenesis and early development. These studies provide taxonomical, developmental, and ecological knowledge, which can be used for the preservation of fish resources and for increasing their proliferation through seed production in different ways (Song & Choi, 2000).
Epinephelus akaara is a fish that belongs to the Perciformes order and Serranidae family, with 12 genera and 27 species found in Korea. Epinephelus akaara is distributed in the south coast of Korea, Jeju-do, Japan, and China (Kim et al., 2005). The Serranidae are currently an economically important taxonomic group, with high industrial value, and several studies on the technical development of their stock production have been done (Heemstra & Randall, 1993; Froese & Pauly, 2014).
Domestic studies of the Serranidae include studies that have been performed on maturation and sex-reversal of the red spotted grouper, Epinephelus akaara (Lee et al., 1998); on the live food habits (Lee & Hur, 1998), time of feeding (Lee & Hur, 1997), changes of egg quality (Lee et al., 1997), and early life cycle of the sevenband grouper, Epinephelus septemfasciatus (Park et al., 2014). Besides these, studies on the skeletal (Park et al., 2015), and egg development; and the effects of water temperature and salinity (Yang et al., 2007; Cho et al., 2015) have also been conducted. Overseas studies include studies on egg development and on morphological development of larvae and juveniles of the sevenband grouper, Epinephelus septemfasciatus (Kitajima et al., 1991), that included studies on deformities in larvae and juveniles (Nagano et al., 2007), their spawning habits, and early life cycle (Ukawa & Higuchi, 1966).
Studies on early life history are very useful for distinguishing between similar fish species, and for determining their relationships. A number of developmental traits provide significant data for elucidating taxonomic relationships (Blaxter, 1974; Balon, 1985). Epinephelus akaara is known to have a characteristic extension of rays in the dorsal and ventral fins at the early larval stage (Ukawa & Higuchi, 1966; Song & Noh, 1998). Although it is easily distinguished from larvae and juveniles of other marine fish, its morphology is similar to that of other Serranidae family larvae, which makes species identification of this taxonomic group difficult. Therefore, this study aims to provide information that might be useful for taxonomic research by investigating egg development, and the morphological development of larvae and juveniles of Epinephelus akaara.
MATERIALS AND METHODS
Fifty adult Epinephelus akaara fish (20 females and 30 males) were used in this study. They matured in June 2013 (37.9±2.99 cm in total length and 888.7±244.7 g in weight). These fish were selected from 200 fish caught from the coast of Geomun-Island, Yeosu-si, Jeollanam-do using fish trap in September 2012 and grown in a fish holder (7×7×5 m). For the feed, combination of horse mackerel, squid, and oyster was provided twice a day at 1–2% of fish weight. To induce spawning behavior, 100 µg/kg of LHRHa was injected into the fish on the dorsal muscle of the lower side of the first ray and the fish were kept in a small-sized fish holder (2×2×2 m).
Fertilized eggs were kept in a 1,000 mL glass beaker and the temperature of breeding water was maintained at 22.0–23.0°C (mean 22.5°C) and salinity at 32.5–33.5% (mean 33.0%) until hatching. Half the breeding water was replaced 5 times each day. Thirty eggs were retrieved randomly and their size was measured with a scale of 0.01 mm, using a phase-contrast microscope (Leica DE DM750, Germany). Egg development was monitored under a stereoscopic microscope (Nikon SMZ18, Japan) and photographs were also taken.
Newly hatched larvae were kept in a circular water tank made of polypropylene (PP) and grown using the intensive pond system under mild ventilation with aeration and liquefied oxygen. As food supply for larvae, 5–10 Brachionus rotundi-formis per mL were provided for 30 days after yolk absorption, and they were supplemented every 6 h to maintain the density. Combination of Brachionus rotundiformis and Artemia sp. were provided from day 30 to 50, and assorted feed (Lovelarva, Japan) was provided after day 50. For studying the morphological development of larvae and juveniles, 10 individuals were retrieved immediately post hatching and anesthetized using an anesthetic (MS-222, Ethyl 3-amino-benzoate methanesulfonate, Sigma Aldrich Co., St. Louis, USA), after which each region of the fish body was measured at the scale of 0.01 mm and observed under a stereoscopic microscope. Morphological developmental stages of larvae and juveniles were classified according to Kendall et al. (1984).
RESULTS
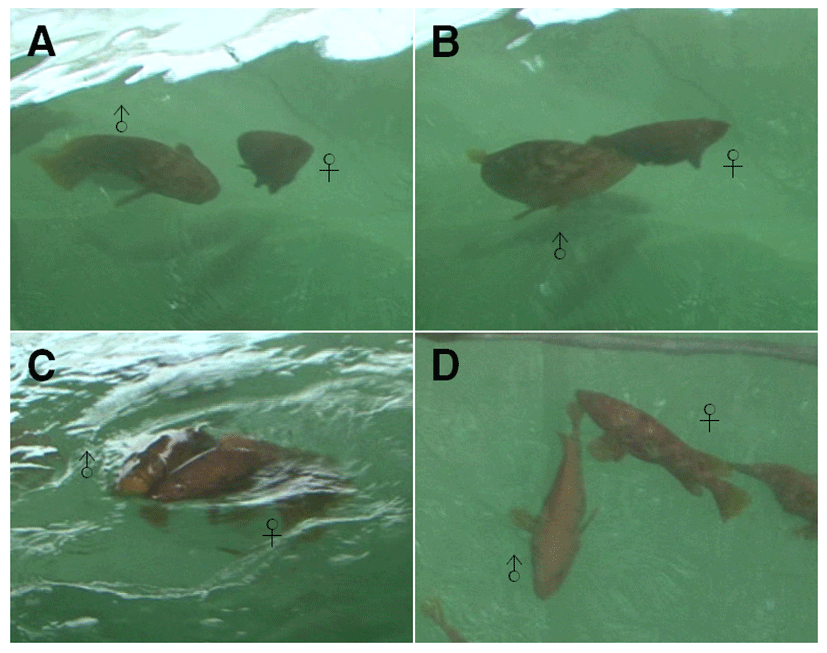
The spawning behavior of Epinephelus akaara was divided into 4 stages as shown in Fig. 1. During the spawning period, males showed nuptial coloration on their head in a spotted pattern, which became fainter towards the tail. Females had an inflated abdomen and the area around the gonopore was red and protruded. Males chased after females consistently, and attacked any other males that approached them (Fig. 1A). Males stimulated females’ abdomen using their head and pushed the females to the surface of the water (Fig. 2B). Males and females moved up to the surface of the water with their bodies attached and swam in circles, while spawning and ejaculation occurred simultaneously (Fig. 1C). After spawning, males and females were separated and initiated spawning again (Fig. 1D).
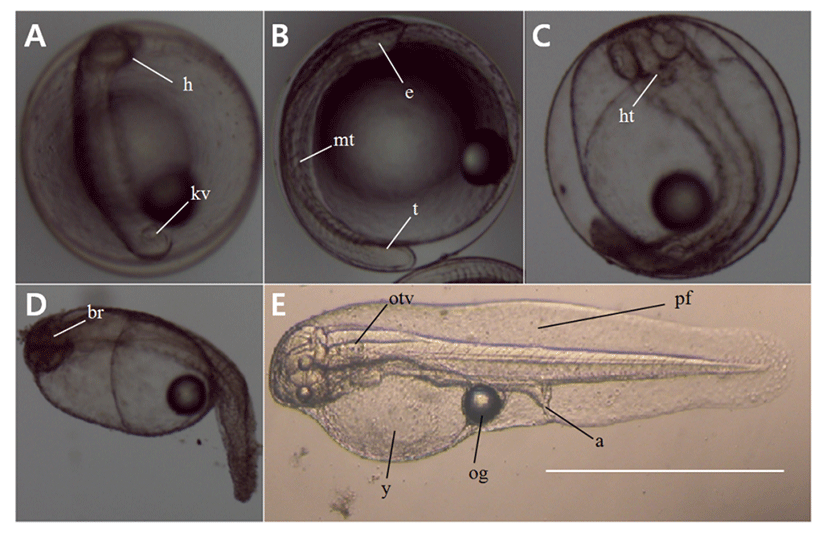
The eggs were 0.71–0.77 mm in size (mean 0.74±0.02 mm, n=30), had one oil globule, and were colorless, transparent, and free pelagic eggs. Fertilized eggs were transferred to the lab immediately after spawning and were observed starting from the 12 h time point post fertilization. We found that at the stage of the embryonic period, the head was formed and a Kupffer’s vesicle had also formed at the end of the tail (Fig. 2A). Kupffer’s vesicle disappeared at 20 h post-fertilization and the embryo began to move; eyes were formed on the head and a primordial fin was present on the tail (Fig. 2B). At the time point 25 h post-fertilization, the embryo started moving actively, the heart had formed, and the heart beat at this stage with the rate of 65–72 beats per min (Fig. 2C). Hatching began at 27 h post-fertilization, as the embryo broke out of the egg membrane with its tail first (Fig. 2D–E).
Newly hatched pre-larvae were 2.02–2.17 mm in size (mean 2.10±0.11 mm) with their mouth and anus not opened yet, and the whole body covered with a membrane fin. The newly hatched pre-larvae had a yolk and one oil globule, did not have the ability to swim and adhered to the bottom or the wall. The digestive tract within the body had developed as a straight line, and the number of myotomes was 23–25 (Fig. 3A).
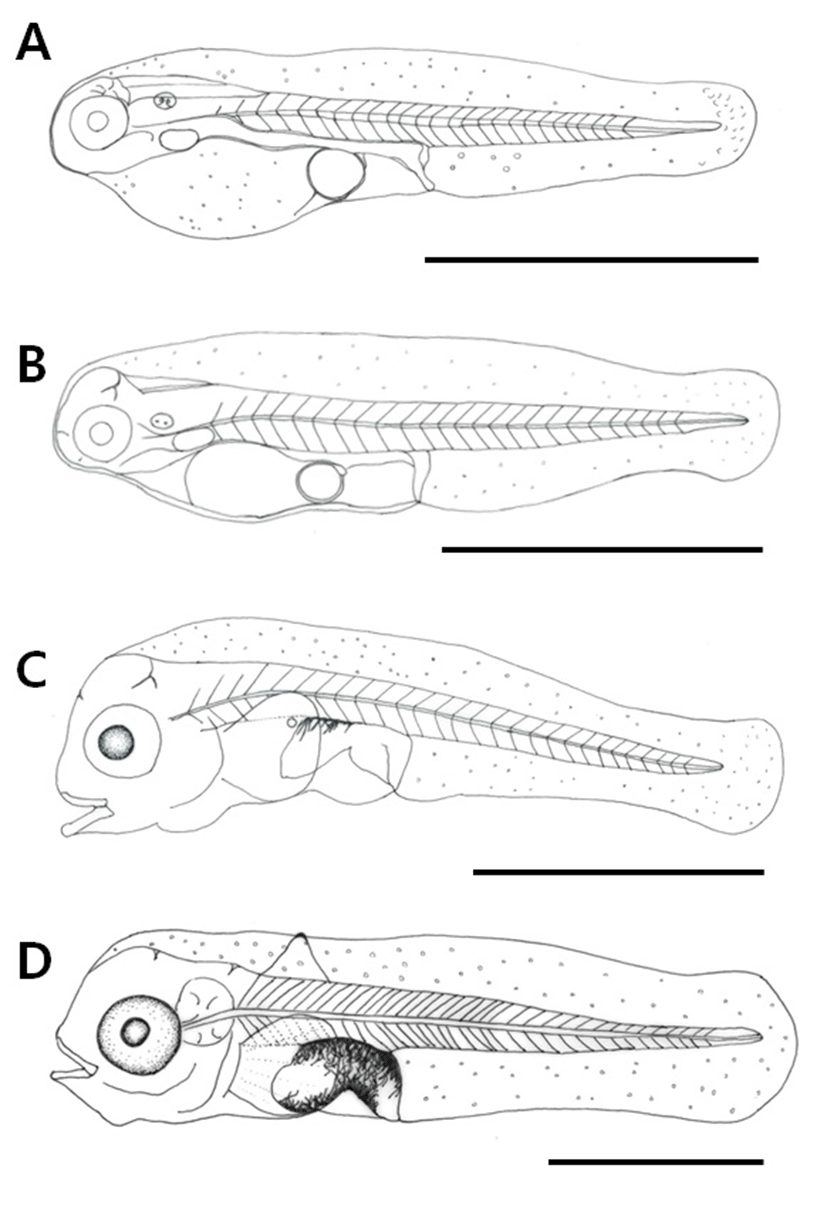
Pre-larvae at 2 days post hatching were 2.21–2.30 mm in total length (mean 2.26±0.06 mm), and the membranous fin was divided from the anus. The sizes of the yolk and the oil globule had reduced. A caudal fin had adopted the shape of a fan, and was actively moving (Fig. 3B).
At 4 days post hatching, the pre-larvae reached 2.43–2.54 mm in length (mean 2.49±0.08 mm) and entered post-larval stage with the entire yolk being absorbed. The mouth and anus were opened, and only a trace of the oil globule remained. The membrane fin that was covering the entire body got connected from the upper head to the lower abdomen, and a round pectoral fin was formed. The digestive tract which had been in a straight line changed into a helical form, and branch-shaped black chromophores were deposited at the top. Pigments were deposited on the lens in the center of the eyes. The number of myotomes increased to 26–28 (Fig. 3C).
Post-larvae at 9 days post hatching were 3.30–3.58 mm in length (mean 3.44±0.20 mm) and 6 rays were formed on the round pectoral fin, while 1 ray was formed on the dorsal fin. Branch-shaped black chromophores, which were previously deposited only above the digestive tract, got distributed more widely on the body, and could be seen below the abdomen also. Pigments were deposited all over the eyes including the center. The anus constituted 37.4% of the body length, reaching almost the center of the body (Fig. 3D).
Post-larvae at 15 days after hatching were 3.88–4.07 mm in length (mean 3.98±0.13 mm), and 1 ray that had formed on the dorsal fin was extended. Two rays were formed on the ventral fin and 8 rays on the caudal fin. The membrane fin was connected from the dorsal fin ray to the anus. Black chromophores were deposited on the caudal peduncle and also at the end of the dorsal and ventral fin rays in a spotted pattern (Fig. 4A).
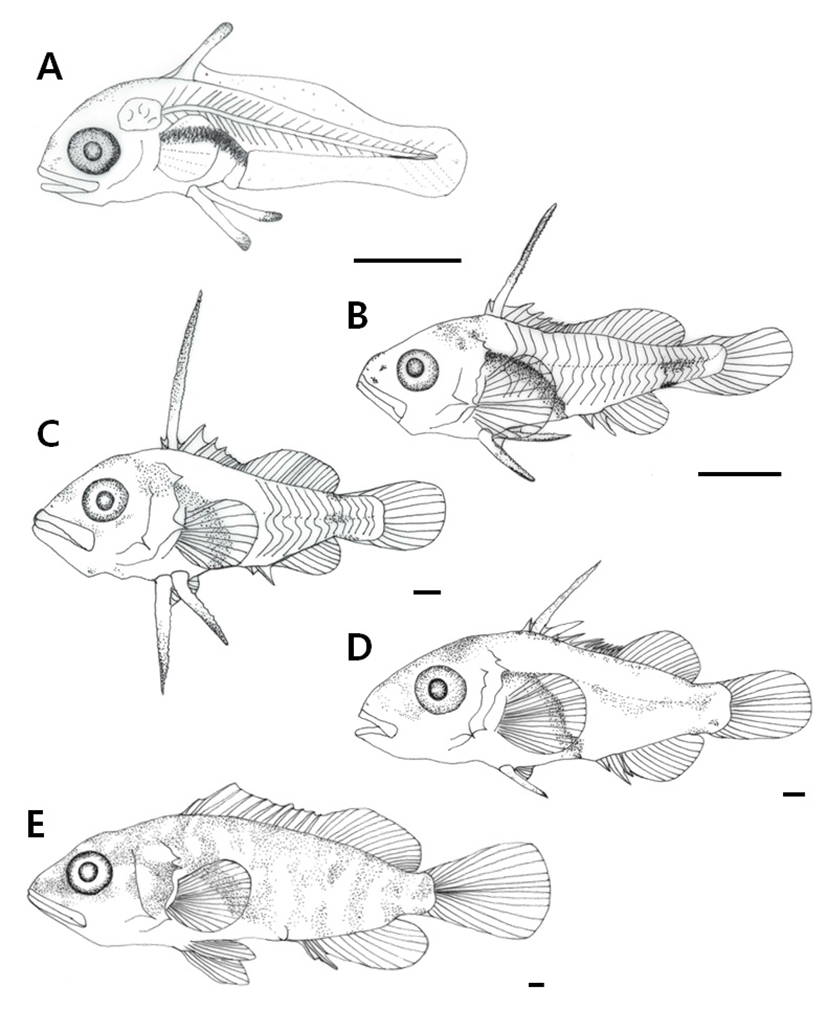
Post-larvae at 25 days post hatching were 4.11–6.32 mm in length (mean 5.22±1.56 mm) and 2 bones were formed on the operculum. The membrane fin was divided into dorsal, ventral, anal, and caudal fins. Black chromophores were deposited on the front mouth, upper head, and the center of the caudal peduncle. The number of fin rays was increased to 25 in the dorsal fin, 12 in the pectoral fin, and 14 in the caudal fin, and 10 rays were formed on the anal fin (Fig. 4B).
Post-larvae at 34 days after hatching were 12.6–17.1 mm in length (mean 14.9±3.18 mm) and the bones formed on the operculum were increased to three. One ray on the second dorsal fin and 2 rays on the first ventral fin were extended, and both ends resembled the shape of a sawtooth. Black chromophores were widely deposited on the head and also on the operculum in a spotted pattern. The number of rays in each fin increased to 26 in the dorsal fin, 12 in the anal fin, and 4 in the ventral fin. At this stage, the position of the anus was at 47.8 % of the body length, proximal to the center of the body (Fig. 4C).
Post-larvae at 46 d after hatching were 19.2–22.5 mm in length (mean 20.9±2.3 mm) and the extended rays of the second dorsal fin and the first ventral fin were reduced in length. Black chromophores were deposited on the center of the body and on the back in a spotted pattern. The number of rays in each fin had increased to 28 in the dorsal fin, 6 in the ventral fin, 17 in the pectoral fin, and 18 in the caudal fin. At this stage, the position of the anus was at 50.0% of the body length, thereby right at the center of the body (Fig. 4D).
Post-larvae at 55 d after hatching were 31.9–35.2 mm in total length (mean 33.6±2.33 mm). Red color was deposited on the entire body and black chromophores were deposited in a spotted pattern. As the number of rays in the ventral fin increased to 8, the number of fin rays, body color, and morphology was similar to that in the adult fish, which led to the juvenile stage. At this stage, the position of the anus was at 49.4% of the body length, proximal to the center of the body (Fig. 4E).
DISCUSSION
Fertilized eggs of Epinephelus akaara are globular-shaped, colorless, and transparent free pelagic eggs with one oil globule. Other marine fish that have free pelagic eggs include Paralichthys olivaceus (Han & Kim, 1997), E. bruneus (Lee & Go, 2003), E. septemfasciatus (Park et al., 2014), Oplegnathus punctatus (Park et al., 2015), O. fasciatus (Koh & Kim, 1992), Acanthopagrus schlegelii (Kim, 1970) and Scomber japonicus (Park et al., 2008), all of which have eggs with one oil globule. Although fertilized eggs with such characteristics are difficult to classify morphologically, differences were observed in the egg sizes, the number of oil globules, as well as in the presence or absence of black chromophores.
The egg size of Epinephelus akaara was 0.71–0.77 mm (mean 0.74±0.02 mm), which was similar to 0.70–0.77 mm reported by Ukawa & Higuchi (1966) and slightly different from the 0.71–0.82 mm size reported by Song & Noh (1998). The egg sizes can differ depending on maturity level, therefore comparative studies on the hatching rate, and egg and larval sizes that take into consideration the maturity level of the fish are further required. The egg size of Epinephelus akaara is smaller than the observed egg sizes for E. septemfasciatus (mean 0.85 mm ) (Park et al., 2014), E. bruneus (mean 0.90 mm) (Lee & Go, 2003), Nassau grouper, E. striatus (mean 0.92 mm) (Allyn et al., 1992), and for the Humpback grouper, Cromileptes altivelis (mean 0.83 mm) (Bulanin et al., 2005), all of which belong to the same family. This shows that other fish of the Serranidae family display differences in their egg sizes, that enables differentiation between these fish.
Water temperature is an environmental factor that affects the development of larvae and juveniles, determines the hatching time for fertilized eggs, and directly affects organogenesis from the embryological aspect (Hempel, 1979; Byun et al., 2007). The time required for hatching in Epinephelus akaara varied with water temperature; it was 27 h at the mean water temperature of 22.5°C in the present study, 23–25 h at 25.1–27.0°C as reported in Ukawa & Higuchi (1966), and 25.5 h at the mean water temperature of 25.0°C from Song & Noh (1998). Moreover, the hatching time is 48 h for E. septemfasciatus (Park et al., 2014) at water temperature of 21.5–23.5°C (mean 22.0°C), 32 h for E. bruneus (Lee & Go, 2003) at mean water temperature of 25.0°C, 20 h and 10 min for Humpback grouper (Bulanin et al., 2005) at water temperature of 27.0–28.0°C, and 27–29 h for Nassua grouper (Allyn et al., 1992) at water temperature of 23.0–30.0°C, showing differences between species. Fish of the Serranidae family showed a tendency of decreasing hatching time at higher water temperature. Differences in time at similar water temperature are most likely a characteristic of the species, but most showed a close relationship with water temperature. Such tendency is also observed in various kinds of fish including Girella punctate and G. melanichthys (Oh et al., 2010), halibut (Kim et al., 2010), Gadus macrocephalus (Lee et al., 2007), mackerel (Hwang et al., 2008), Takifugu pardalis (Han & Cho, 2007), and Microstomus achne (Byun et al., 2009).
We found the larval size of Epinephelus akaara to be 2.02–2.17 mm in length (mean 2.10±0.11 mm), while Ukawa & Higuchi (1966) reported it to be 1.45–1.56 mm and Song & Noh (1998) reported a mean of 1.65 mm, showing that there are differences between the studies. Total length of larvae was found to be 1.74 mm (mean) in E. septemfasciatus, 1.30–1.40 mm in E. fasciatus (Kawabe et al., 2009), 1.86 mm (mean) in Humpback grouper (Bulanin et al., 2005), and 1.70–1.80 mm in Nassau grouper (Allyn et al., 1992), which were all shorter than the length of Epinephelus akaara larvae. Length of E. bruneus larvae was 2.02 mm (Lee & Go, 2003), which was in a similar range.
Fish of the Serranidae family undergo morphological changes at the post-larval stage. The onset of these changes depends on the time when the second ray of the dorsal fin and the first ray of the ventral fin begin to extend. In the present study, the onset of morphological changes in Epinephelus akaara was observed to be on the 15th day after hatching, and when the length was 3.98 mm. Similar results were reported by Ukawa & Higuchi (1966), where the onset of morphological changes were observed 15 days after hatching and when the length was 4.05 mm and by Song & Noh (1998) who reported it to occur between 14–17 days after hatching. Although there were differences in total length, the timing was similar. Morphological changes of E. septemfasciatus (Park et al., 2014) occurred at 8 days after hatching, which was earlier than that of Epinephelus akaara. The onset of morphological changes, and larval size are likely to differ depending on feeding, breeding conditions, and development.
The number of days taken to reach the juvenile stage was 55 days post hatching and the size of the juveniles was 31.9–35.2 in total length (mean 33.6±2.33 mm) in Epinephelus akaara. It was 61 d post hatching and the mean length was 32.9 mm for E. septemfasciatus (Park et al., 2014). Thus, their sizes were similar but Epinephelus akaara needed a shorter time for attaining the juvenile stage. E. bruneus (Lee & Go, 2003) attained the juvenile stage 50 d after hatching and reached 10.5 mm in total length; E. fasciatus (Kawabe et al., 2009) required 30 d post hatching and attained a length of 12.4 mm; Nassau grouper (Allyn et al., 1992) required 40 d after hatching and attained a mean length of 13.5 mm. Compared to these fish, Epinephelus akaara took longer time and had a bigger size (Table 1).
Several studies have been performed on the technical development of the domestic Serranidae stock production in Korea. However, not many studies have been performed on egg development and morphological development of larvae and juveniles. Further studies on the early stages of the life cycle of the Serranidae family are required to promote the taxonomical research on larvae and juveniles, and to elucidate the causes of characteristics such as deformities, large-scale mortality, and cannibalism that occur during breeding.

