INTRODUCTION
The fish of family Serranidae are very important species at aquaculture industry in the world and marketable in South Korea, Japan, China, and Southeast Asia. Especially, red spotted grouper, E. septemfasciatus, was known as most expensive fish among those. So, many fish farmers were more interested in aquaculture of this species and recently, mass seedling production technology was developed in South Korea. Korea is located in the temperate climate zone. So often it causes mortality of fish in the summer and winter seasons at sea cage fish culture, because which do not avoid from sudden environmental changes in an enclosed rearing condition.
Generally, the major stress factors affecting fisheries include physical factors (e.g., water temperature, salinity, culture density) and chemical factors (e.g., formalin, sanitizers; Singley & Chavin, 1971; Fryer, 1975; Wedemeyer & Mcleay, 1981). Among these factors, seawater temperature is the most important factor for various aspects of aquaculture, including fish growth, survival, metabolism, and immunity (Ishioka, 1980; David & Parker, 1990; Ryan, 1995). Hematological factors have been used as indicators of the physiological stress responses of fish to changes in water temperature (Adam, 1990; Santos & Pacheco, 1996; Cataldi et al., 1998). Exposure to changes in water temperature results in rapid stress, leading to losses of immunity, increased disease, physiological changes and even mass mortality, so which directly affect productivity and economic loss (Wedemyer & Mcleay, 1981; Hur & Habibi, 2007; Kang et al., 2007).
Red spotted grouper is a species that inhabits the southern coast of South Korea. Accoriding to Froese & Pauly (2014), This species is a temperate fish, and water temperature of habitat is range from 11.7 to 26.3°C. But, there is no experimental work about the temperature limits of Red spotted grouper for reliable aquaculture.
The goal of the present paper is to study the influence of water temperature change upon the survival rate and hematological characteristics of Red spotted grouper at low and high water temperature conditions.
MATERIALS AND METHODS
This experiment was conducted at the South Sea Fisheries Research Institute of the Yeosu in Korea. Experimental fish (body length 15.18±1.24 cm; weight 50.95±10.96 g) were produced artificially at july 2014. Prior to experiments, 400 fishes were acclimatized for four weeks in 1 ton recirculating tank at 12°C and 28°C water temperature condition, respectively. Changes of water temperature was manipulated by water control system, and the experiments were conducted in a running water culture system (5 circulations/1 day) with fasting for 9 days. Meanwhile, the salinity was maintained at 32.1–33.2‰, and the dissolved oxygen level was maintained at 6.8–8.9 ppm.
Two regimes (low and high temperature conditions) of temperature treatments were used for this study. At low temperature condition, the water temperature was decreased by 1°C every 24 h (from 10°C to 4°C, –1°C/d), and at high temperature, the water temperature was increased by 1°C every 24 h increased 1°C every 24 h (from 28°C to 34°C, +1°C/d). Two replicates of each treatment were conducted in a total of 8 water tanks (Polypropylene, ∅ 1.0 m × H 1.2 m) with a each 1 ton capacity; four tanks were used for the mortality experiments, and the other four were used for hematological analysis. For hematological analysis, five fish were randomly selected from each temperature treatment (n=10/replicate).
Cumulative survival rate was calculated every 24 h, and specimens were considered dead when they lost their swimming ability and stopped breathing through their gills for ≥1 min.
Each day, blood was collected from the tail artery of fish (n=5/tank) within 1 min with a heparin-treated syringe (1 mL) without the application of anesthesia, and sampled fish were transferred to a recovery tank, in order to avoid multiple samples from individual fish. The collected blood samples were centrifuged (12,000 rpm, 5 min) to separate the plasma, which was stored at –70°C until the analysis of alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose, total protein (TP), electrolytes, and cortisol. A kit slide was used for the analysis using automatic biochemical analyzer (FUJI DRI-CHEM 3500i; Fujifilm Co., Japan), and plasma cortisol was measured using enzyme-labeled antigen with a cortisol EIA kit (Oxford, USA).
RESULTS
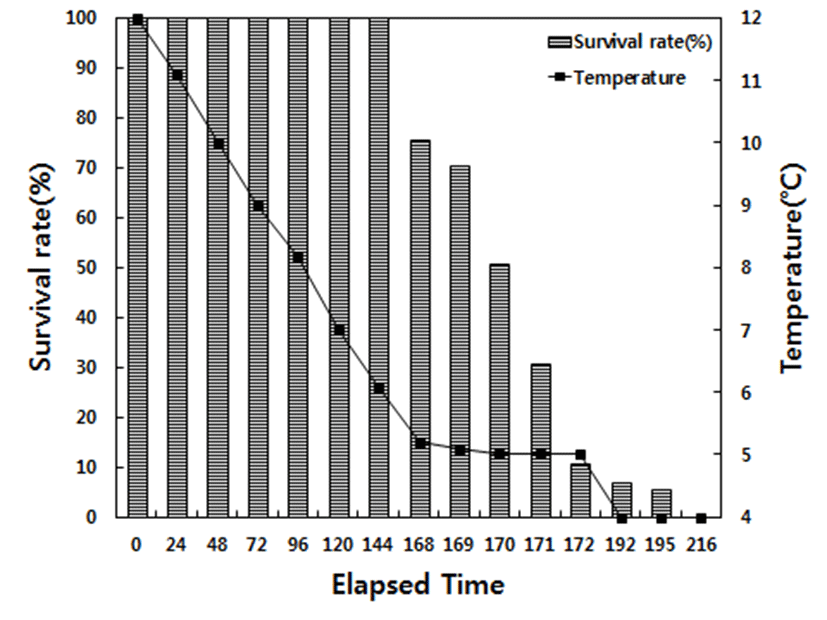
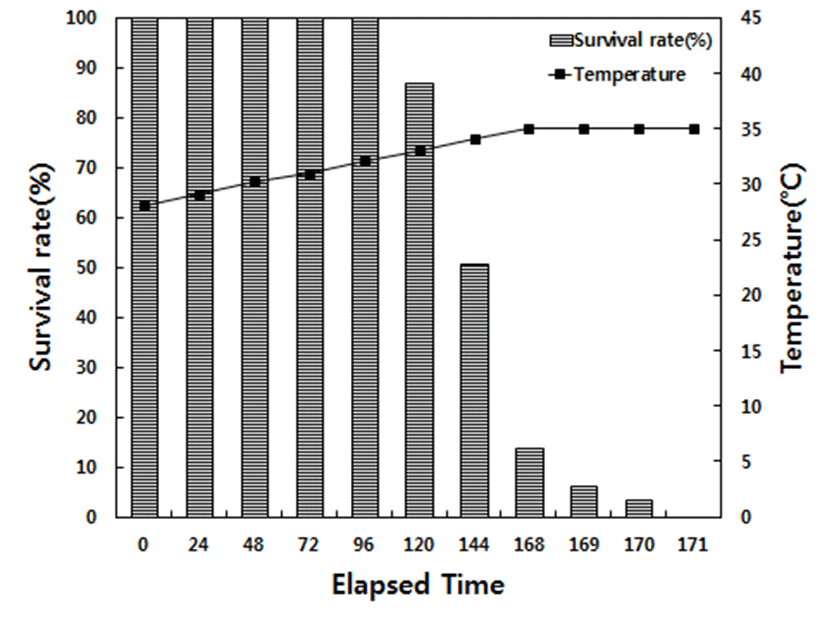
No mortality was observed in the decreasing temperature experiment until 168 h, at which the water temperature had decreased to 5°C in low water temperature, and the survival rate at 168, 169, 170, and 172 h was 75.4 %, 70.3 %, 50.6 %, and 10.5 %, respectively. When the water temperature decreased to 4°C (192 h), the survival rate was only 5.1% survived after 3 h (195 h), and 100% mortality was observed after 24 h at 4°C (216 h). For the increasing temperature experiment at high temperature condition, no mortality was observed until 32°C (96 h), at which the water temperature had increased to the survival rate at 33°C (120 h) and 34°C (144 h) was 86.7 and 50.6%. When the water temperature increased to 35°C (168 h), the survival rate was only 13.5% and 3.2% after two additional hours (170 h); 100% mortality was observed after 3 h at 35°C (171 h; Fig. 1 and Fig. 2).
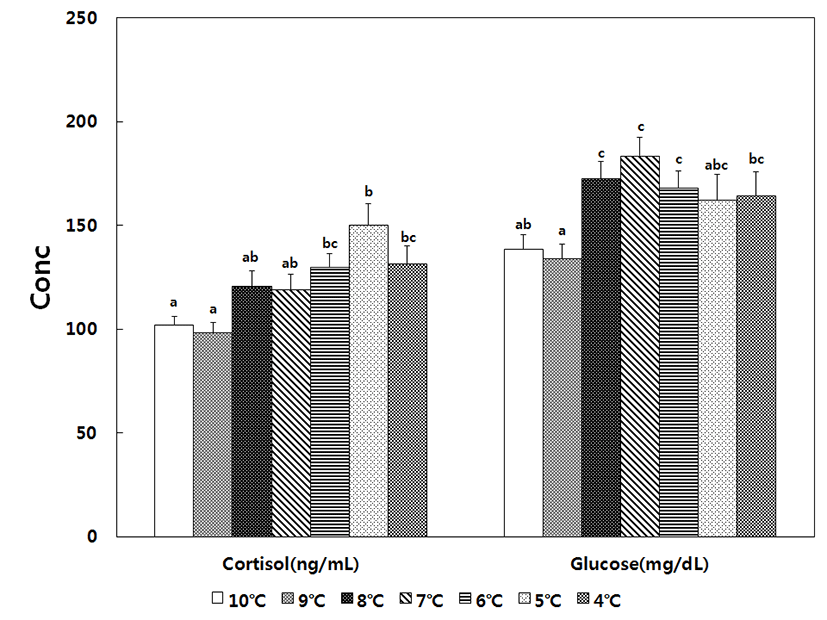
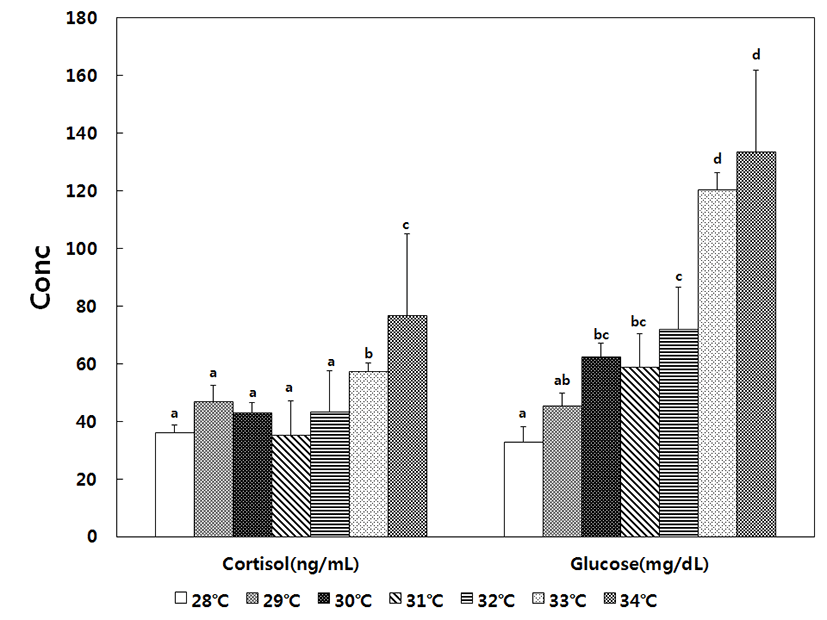
For fish included in the decreasing temperature treatment, plasma cortisol was 101.9 ± 5.4 ng/mL in the 10°C control group, began increasing at 8°C, reached maximum value of 150.1 ± 5.3 ng/mL at 5°C, and slightly decreased to 131.8 ± 5.1 ng/mL at 4°C. Thus, decreases in water temperature significantly increased the level of cortisol until 4°C. The glucose level was 138.6 ± 5.4 mg/dL in the control group, began increasing at 8°C, reached a maximum value of 183.6 ± 8.2 mg/dL at 7°C, and decreased to 168.0 ± 4.6 mg/dL at 6°C, at which point the level was still significantly higher than that of the control group. For fish included in the increasing temperature treatment, the level of cortisol was 36.1 ± 2.6 ng/mL in the 28°C control group, remained stable up to 32°C, began increasing at 33°C (57.3 ± 2.7 ng/mL), and reached a maximum value of 76.7 ± 8.9 ng/mL at 34°C. The glucose level was 32.8 ± 2.5 mg/ dL in the 28°C control group, gradually increased to 72.1 ± 3.7 mg/dL at 32°C, began increasing rapidly at 33°C, and reached the maximum value of 133.6 ± 5.8 mg/dL at 34°C. Therefore, higher levels of cortisol and glucose were observed under conditions of decreasing temperature (Fig. 3 and Fig. 4).
For the fish included in the decreasing temperature treatment, the plasma level of AST was 76.7 ± 8.8 u/L in 10°C control group, began increasing at 9°C, reached a maximum value of 129.6 ± 7.4 u/L at 6°C, and decreased to 114.6 ± 4.5 u/L at 4°C. When compared to the AST levels of the control group, the values were significantly higher from 9 to 4°C. The level of ALT was 18.6 ± 0.3 u/L in the 10°C control group, significantly increased in a manner similar to AST, and reached a maximum value of 24.5 ± 2.0 u/L at 4°C. Meanwhile, the plasma level of total protein was 2.8 ± 0.1 g/dL in the control group, increased to 3.4 ± 0.2 g/dL at 7°C, began decreasing at 6°C, and reached 2.4 ± 0.1 g/dL at 4°C, which was lower than that of the control group.
For the fish included in the increasing temperature treatment, the plasma level of AST was 45.3 ± 10.4 u/L in 28°C control group, significantly increased to 61.0 ± 7.4 u/ L at 33°C, and reached 88.2 ± 12.8 u/L at 34°C, which was significantly higher than that of the control group. The level of ALT was 7.4 ± 0.3 u/L in the 28°C control group, remained stable to 31°C, began increasing to 10.7 ± 1.7 u/L at 32°C, and reached 13.3 ± 0.8 u/L at 34°C, which was significantly higher than the control group. Meanwhile, the level of total protein ranged from 2.3 ± 0.2 g/dL to 3.1 ± 0.4 g/dL, with no statistical difference between groups (Table 1).
For fish included in the decreasing temperature treatment, the plasma levels of Na+, K+, and Cl– ranged from 157.0 ± 6.9 mmol/L to 195.2 ± 11.8mmol/ L, from 3.0 ± 0.2 mmol/L to 3.6 ± 0.2 mmol/L, and 143.1 ± 7.2 mmol/L to 162.2 ± 3.6 mmol/L, respectively, with no statistical differences between groups. For fish included in the increasing temperature treatment, the plasma level of Na+ ranged from 155.3 ± 8.5 mmol/L to 180.7 ± 1.9 mmol/L, with no statistical difference was between groups. The plasma level of K+ was 2.2 ± 0.2 mmol/L in the 28°C control group, increased significantly to 3.4 ± 0.3 mmol/L at 33°C, and decreased to 3.1 ± 0.2 mmol/L at 34°C, at which point it was still significantly higher than that of the control group. Meanwhile, the plasma level of Cl– was 149.6 ± 5.6 mmol/L in the 28°C control group, remained stable to 32°C, increased to a maximum value of 178.5 ± 3.9 mmol/L at 33°C, and decreased slightly 166.5 ± 4.9 mmol/L at 34°C, at which point it was still significantly higher than that of the control group (Table 1).
DISCUSSION
Normally, cold-blooded animals like fish are able to adapt to the external environment; however, the physiological activity level of fish rapidly decreases outside of their critical water temperature, thus impairing their health (Barton & Iwama, 1991). Temperature tolerance allows organisms to adapt to environmental changes, and each organism has an optimal temperature range (Shamseldin et al., 1997). However, the average coastal water temperature of South Korea is lower than the optimal water temperature for red spotted grouper, which may result in reduced intake rate, deteriorated nutrition status, retarded growth, and even death (Park et al., 2011). The water temperature in South shore areas ranges from 5 to 33°C, so fish that are farmed in immobile cages often experience environmental assaults, owing to changes in water temperature during winter and summer. In particular, the red spotted grouper, which is a temperate fish, can undergo mass mortality when the fry produced during the summer are exposed to lower winter temperatures. Therefore, it is imperative to identify appropriate growth conditions for yearlong culturing, in order to increase the competitiveness of farming, and to conduct physiological and biochemical research to identify the critical water temperatures for seed cultivation under stable conditions.
When we investigated the survival rate of red spotted grouper at low water temperatures, we observed that mortality increased from 24.6% at 5°C (168 h) to 49.4% (170 h) and further to 94.9% at 4°C, eventually reaching 100% mortality at 216 h. At higher temperatures, we found that mortality increased from 13.3% at 33°C (120 h) to 49.4% (168 h) and further to 96.8% at 35°C (170 h), eventually reaching 100% mortality at 171 h. Thus, the findings of the present study indicate that red spotted grouper reach their physiological limits at 4°C and 35°C, owing to overwhelming stress, and die as a result. Park et al. (2016) observed 100% mortality in sevenband grouper at 5°C; thus, red spotted grouper seem to exhibit higher resistance to low temperatures. The critical water temperatures reported by the present study are 5 and 34°C; however, we changed water temperature over a relatively short period. Therefore, the survival rate is expected to be higher in the field, where the fish likely experience less drastic changes in water temperature.
In fish, behavioral responses are considered the primary response to stress (Schreck & Moyle, 1990). For example, Rainbow trout, Oncorhynchus mykiss reported that swimming ability increases with decreasing water temperature until 10°C and that motility decreased below 5°C (So et al., 2008). In the present study, we observed that swimming ability increased at 8°C and that as temperatures decreased below 7°C, mobility gradually decreased and fish bodies began to rub each other. At 5°C, fish lost both swimming ability and balance, and the only observed movement was that the opening and closing movement of the gill covers. Then, at 4°C, the secretion of mucus increased, fish bodies became stiff, and the opening and closing movement of gill covers became more rapid, in agreement with previously reported observations in sevenband grouper at 7°C (Park et al., 2016) and in flatfish and Paralichthys olivaceus (Hur & Habibi, 2007). Meanwhile, in fish included in the increasing temperature treatment, no significant changes were observed in swimming ability until the water temperature reached 34°C, at which point the opening and closing movement of gill covers became more rapid and swimming ability decreased. Then, at 35°C, we observed an increase in mucus secretion, bubbles formed at the surface of the water tank, and 100% mortality occurred.
Fish normally produce cortisol to cope with the stress of changes in water temperature and to maintain growth and survival, and cortisol levels are known to correlate with environmental factors, such as water temperature and salinity, as well as with fish size, type, and development stage, owing to the rapid exchange between catecholamine and corticosteroid (Barton & Iwama, 1991; Mommsen et al., 1999). Under stress, the typical level of cortisol in salmon ranges from 40 to 200 ng/mL, and the level can be even higher in other species. In addition, cortisol levels can also become elevated in response to external stress (Pickering & Pottinger, 1989; Yoon et al., 2016). For example, in mullet (Chelon haematocheilus), the cortisol level was 48.5 ± 4.8 ng/mL in a control group maintained at 10°C, and the level increased to 218.0 ± 40.6 ng/mL when the temperature was lowered to 0.5°C (Kang et al., 2007). A similar trend was observed in the present study, since the cortisol level increased from 101.9 ± 5.4 ng/mL in the control group at 10°C to 150.1 ± 5.3 ng/mL when the temperature was lowered to 5°C. In contrast, black porgy (Acanthopagrus schlegeli) exhibited a cortisol level of 6.5 ± 0.7 ng/mL when maintained at 20°C and a level of 10.6 ± 29.9 ng/mL at 30°C (Choi et al., 2006). A similar trend was observed in the present study, since the level of cortisol in the control group was 36.1 ± 2.6 ng/mL, and the level increased to 76.7 ± 8.9 ng/mL when the temperature was increased to 34°C.
Therefore, increased cortisol levels may result from sudden physiological changes, owing to exposure to water temperatures beyond their tolerance level, such as below 5°C or above 34°C in the red spotted grouper. Fish that have become adapted to high temperatures during summer have been reported to exhibit lower tolerance to low temperatures, and fish adapted to low temperatures during winter have been reported to exhibit lower tolerance to high temperature, so the level of cortisol has been reported to increase proportionally to the water temperature stress (Horning & Pearson, 1973; Davis et al., 1990; Parihar et al., 1996; Parihar et al., 1997; Chang et al., 1999). In the present study, the cortisol level of the 10°C control group was ~2.8 times higher than that in the 28°C control group and fish at 34°C. As a temperate fish, red spotted grouper might not be physiologically stable at 10°C, and its ability to adapt to water temperature changes may be lower for low water temperatures than for high water temperatures. In addition, the loss of physiological adaptation caused by stress is assumed to be higher at low water temperature. Furthermore, hypoxia stress has also been reported to cause fish mortality (Jeong et al., 2014). Therefore, further investigation is warranted.
Fish require glucose as an energy source to overcome unstable physiological statuses and to maintain homeostasis. Along with cortisol, the glucose level of teleost fish has generally been reported to increase upon exposure to the stress, owing to the increased activation of enzymes involved in gluconeogenesis (Davis et al., 1985; Barton & Iwama 1991), as in mullet (Mugilcephalus spp.; Chang & Hur, 1999), pejerrey (Odontesthes bonariensis), and sevenband grouper (Park et al., 2016). Similarly, in the present study, the plasma glucose level was 138.6 ± 5.4 mg/dL in fish from the 10°C control group and increased to 183.6 ± 8.2 mg/dL in fish exposed to 7°C, whereas the plasma glucose level was 32.8 ± 2.5 mg/dL in fish from the 28°C control group and increased to 133.6 ± 5.8 mg/dL at in fish exposed to 34°C. Thus, the increased need for brain metabolism and homeostasis maintenance, owing to changes in the water temperature, are thought to result in elevated glucose levels, as a source of energy. The use of glucose was higher under the decreasing temperature treatment than under the increasing temperature treatment, thus resulting in higher levels of glucose in fish exposed to lower temperatures.
Blood AST and ALT levels are indicators of liver function in liver and spleen cells as aminotransferase, and their activation is elevated upon exposure to stress from water temperature changes and hypoxia, which indicates damage to liver cells (Pan et al., 2003). Similar results were observed in the present study, in which the levels of both AST and ALT increased in response to decreasing and increasing temperature. Prolonged exposure to low or high water temperature is thought to result in cell damage, and greater damage seems to occur as a result of exposure to low temperatures.
In addition, total protein level is commonly used in the diagnosis of fish health, nutritional status, and disease, and it typically ranges from ~4 to ~7 g/dL (Turner, 1937; Ozaki, 1978; Yanagisawa & Hashimoto, 1984), although the level observed in the present study was slightly lower. When fish are under stress, the primary response is increased plasma cortisol, with increases in protein level as a secondary response (Davidson et al., 2000). However, Ishioka (1980) observed decreased levels of total protein in fish under stress, and in the present study, the no significant changes were observed.
Electrolytes are secreted by fish under stress in order to overcome the confusion; however, this results increases the organism’s energy requirement (Nolan et al., 1999; Choi et al., 2007). In addition, the level of metabolism is decreased, and blood electrolyte levels are also affected by external factors (Ishioka, 1980; Robertson et al., 1987, 1988; Davis & Parker, 1990). In the present study, the decreasing temperature treatment failed to induce any significant changes in electrolyte levels, whereas the increasing temperature treatment resulted in increased Cl– levels, as previously reported by Do et al. (2014). However, no significant changes were observed for Na+ or K+. In response to changes in water temperature, the ability of fish to control osmotic pressure is thought to become impaired, leading to instable electrolytes.
Therefore, the plasma cortisol and glucose levels of fish under the decreasing temperature treatment rapidly increased at 5 and 7°C, respectively, and fish lost swimming ability and exhibited rapid gill cover movement below 7°C. Meanwhile, the plasma cortisol and glucose levels of fish under the increasing temperature treatment rapidly increased at 34°C, respectively, and fish lost swimming ability and exhibited increased mucus secretion at 33°C.

