INTRODUCTION
Pacific oysters Crassostrea gigas are culturing by many countries in the world and a total of 637,670 tonnes with global production has been reported (FAO, 2009). In Korea, oyster is the economically most important aquaculture shellfish species occupying 40% of the world culture products and being exported to 20 countries. However, recently oyster production has declined due to diseases such as herpesvirus, oyster velar virus and Marteilioides chungmuensis and environmental pollution. Moreover intensive farming and shortage of natural feed planktons has deteriorated the quality of oyster broodstock and health of spat, consequently massive mortality of seedlings has been frequently observed in many natural spat collection areas. In order to overcome these problems, establishment of artificial seedling production has become imperative. For the production of healthy spat, conservation of genetically superior adult oysters as well as their larvae is firstly required, subsequently, cryopreservation techniques of each developmental stage in oyster larvae must be developed.
There have been several studies on cryopreservation of eggs and embryos of vertebrate fishes such as medaka Oryzias latipes (Arii et al., 1987) and rainbow trout Oncorhynchus mykiss (Ahammad et al., 1998) etc., but the results have not been reported satisfactory yet. This might be due to the big size of bony fish eggs, too much yolk (Mazur, 1984) and the low impermeability of water a cross the two different membranes–cytoplasmic membrane and yolk membrane (Wallace & Selman, 1990). On the other hand, it has been reported that cryopreservation is holding a greater potential in shellfish than the finfish, because bivalves have smaller embryos and less amount of microlecithal eggs. Moreover, bivalve embryos show a holoblastic cleavage, more favorable to cryoprotectant penetration (Suquet et al., 2012).
Subsequently, several bivalves species have been the subject of cryopreservation research: such as blue mussel Mytilus edulis (Toledo et al., 1989), Pacific oyster Crassostrea gigas (Renard, 1991; McFadzen, 1992; Gwo, 1995; Chao et al., 1997; Naidenko, 1997), Manila clam Ruditapes philippinarum (McFadzen, 1992), hard clam Meretrix lusoria (Chao et al., 1997), pearl oyster Pinctada fucata martensii (Chang et al., 1999), surf clam Spisula sachalinensis (Chang et al., 2001), and arkshell Scapharca broughtonii (Jo et al., 2002). More importantly particularly in Pacific oyster, Paniagua-Chavez et al. (1998) reported that freezing of trochophores was successful and the post-thawed larvae developed more and settled down on substrate as the spats. The scientific literature reviewed by Robles et al. (2009) and Paniagua-Chavez et al. (2000), underlying the problem of embryo survival estimation after cryopreservation. Whereas, Choi & Chang (2003) reported that more than 85% of D-shaped veliger in pearl oyster survived just after post-thaw. Nevertheless, this results could not be applied in practical spat production, since the authors didn't observed the survival rate with time course and further development progress for the post-thawed larvae. To our knowledge there has been an absolute lack of information on the survival and development of post-thawed larvae with the time course reared with food supply.
Therefore, the present study aimed to provide the basic information of frozen-thawed oyster larvae survival, development and growth with time course in indoor rearing condition.
MATERIALS AND METHODS
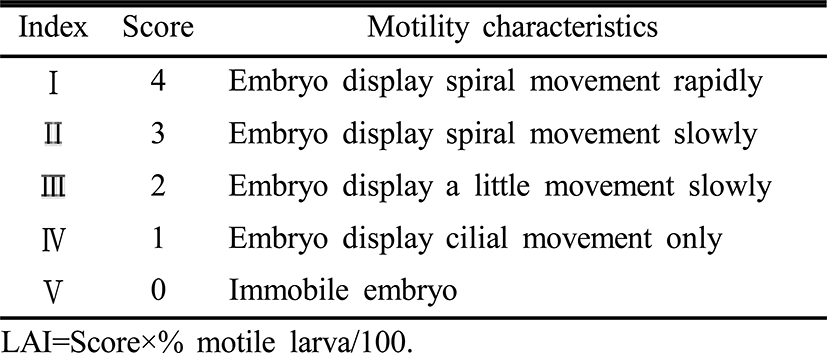
8 individuals of cultured oysters (1-2 years old), averaging shell length of 52.5±4.1 mm, total weight of 62.0±10.1 g and meat weight of 11.1±2.5 g were used for eggs and sperm collection. Gamete specimens were collected from fully matured oysters by stripping method. Obtained eggs and sperm were suspended in each 1 L beaker and fertilized in a ratio of 1 egg to 10 spermatozoa. Six million eggs (35 eggs/ml) were washed through triple meshes of 140 μm, 65 μm, 20 μm MÜller gauze, sterilized with 150 ppm sodium hypochlorite and ultraviolet sterilizer (Jebo, China) and incubated in clean seawater (33 psu) until use. When 50% of larvae reached to trochophore (TCP), D-shaped veliger (DSV) and early umbo veliger (EUV), their developmental stages were determined and then TCP aged 10 hours, DSV aged 4 days, EUV aged 8 days with active motility were subjected to experiments (Fig. 1). Counting of gametes and larvae was duplicated in every time.
Larvae were cryopreserved, according to the protocol, briefly the extender was 0.2 M sucrose prepared by melting in artificial seawater (NaCl 2.7 g, KCl 0.07 g, NaHCO3 0.05 g, CaCl2 0.12 g, MgCl2 0.46 g, Milli-Q water 100 ml) and different cryoprotectant (CPA) concentrations of ethylene glycol (EG) 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 M concentration. The larvae for freezing experiments were permeated in each CPA at room temperature of 20°C, after 10 minutes reaching to the equilibration and transferred into 0.5 ml straw (FHK, Japan). The larvae per straw were 300 individuals in TCP, 200 ind. in DSV and 200 ind. in EUV. Every five straws were used in each experimental group. Larvae were frozen in a program freezer (Samwon Freezing Engineering Co., Korea). The initial temperature was 0°C, the straws were cooled at a rate of –1°C/min from 0 to –12°C and the straws were kept at –12°C for a period of 10 min after seeding at –12°C. Seeding was induced by dipping the straws into liquid nitrogen. The straws were cooled at the same cooling rate from –12°C to until reaching a final temperature of –35°C, which was held for 30 min. Straws were plunged into liquid nitrogen tank (MVE, USA). Straws were thawed in a water bath at 20°C for 30 sec. Protocols for the freezing and thawing used in the present experiment are summarized in Table 1.
|
The survival rate and motility were observed under microscope (×100) and determined by calculating LAI according to Table 2. In observation under the microscope, the ratio of abnormality individuals was calculated (abnormality if the epidermis was damaged for TCP or if the damaged organs were eluted out of the shell for DSV). Each experiment was conducted in triplicate group of treatments.
To evaluate survival rate and LAI with time course by rearing of thawed larvae, following protocol and the appropriate CPA concentration determined in previous freezing step, frozen TCP, DSV and EUV were subject to experiment used. First, frozen larvae were rapidly thawed at 20°C and then eliminated CPA by filtered seawater for 15 min subsequently, reared in 2 L capacity beakers for a week. Every sixty straws were used in each larval group. The larvae per straw were 400 ind. in TCP, 300 ind. in DSV and 300 ind. in EUV and rearing were carried out until the survival of last larva. Rearing seawater was exchanged once a day and thawed larvae were fed 10 ml of Isochrysis galbana with 20,000 cells/ml twice a day.
The significance of differences between mean survival rates, LAI and abnormality rates for each factor were tested by one-way ANOVA and Duncan's multiple range test.
RESULTS
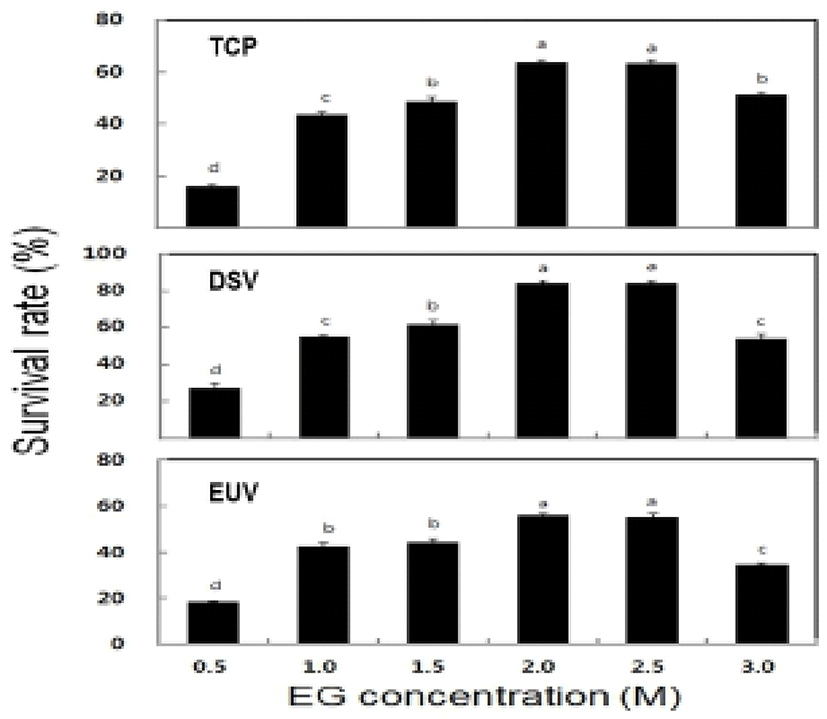
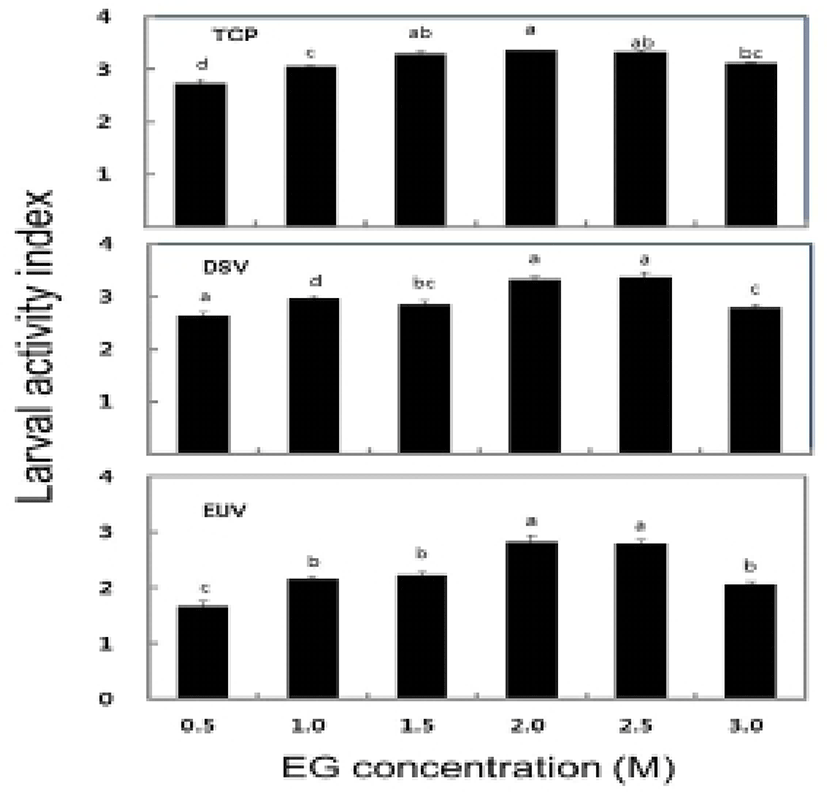
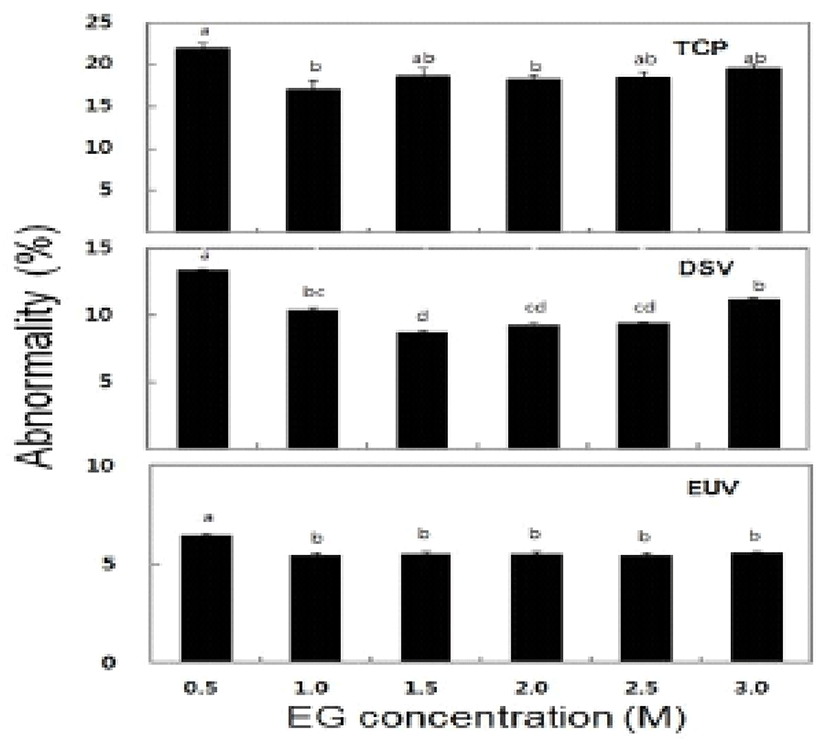
The survival rate of post-thawed larvae were different in three larval developmental stage in six different concentration of EG. The survival rate for TCP, DSV and EUV ranged for 16.3-63.8%, 27.2-84.1% and 18.4-56.3%, respectively. The highest survival rates of 63.8± 1.3% in TCP, 84.1±1.5% in DSV and 56.3±0.6% in EUV were observed at 2.0 M EG concentration (Fig. 2). However, there were no significant differences in the survival rate between the concentrations of 2.0 M and 2.5 M EG. The LAI of post-thawed larvae ranged for 2.7-3.4 in TCP, 2.6-3.4 in DSV and 1.7-2.8 in EUV. LAI at 2.0 M EG was observed 3.4±0.2 in TCP, 3.4±0 in DSV and 2.8±0.1 in EUV (Fig. 3). However, there were no significant differences in LAI between 2.0 M and 2.5 M EG. The abnormality rate of post-thawed larvae were recorded 17.1-21.9% in TCP, 9.3-13.4% in DSV and 5.5-6.5% in EUV in all EG concentrations. Abnormality rate was the highest in 0.5 M EG, but there was no significant difference among all EG concentrations (Fig. 4).
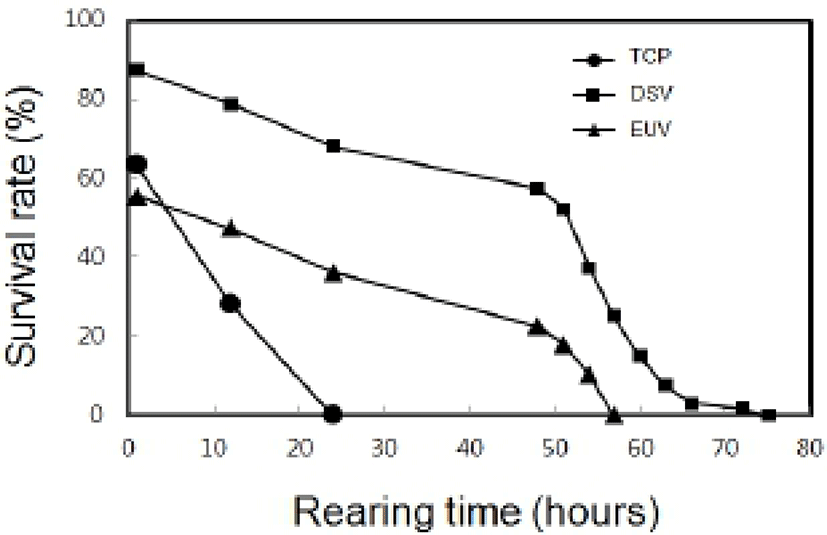
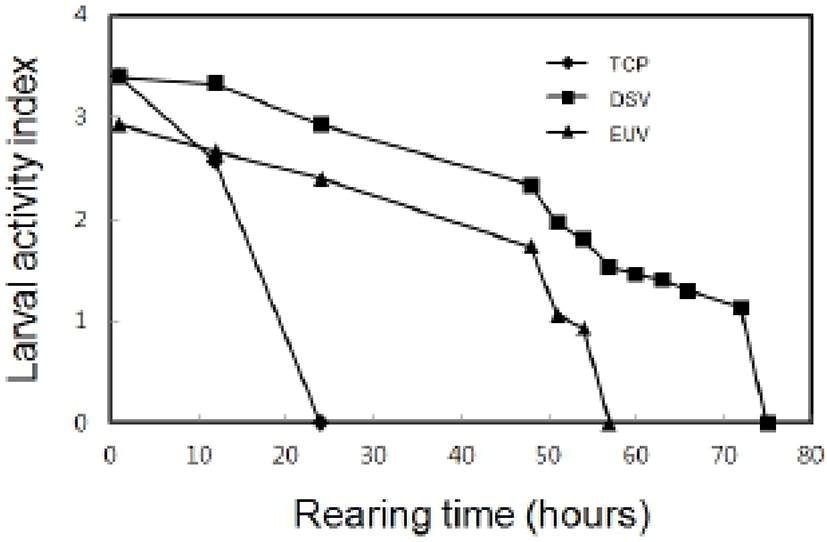
In indoor rearing experiment, the survival rate by the elapsed time in post-thawed larvae was shown as Fig. 5. The survival rate just after thawing in TCP was 63%. However, survival rate of TCP was decreased to 28% after 1 hour and then no TCP was alive within 24 hours. The survival rate of DSV was 87%, and then the survival rate was gradually reduced to 79% within 12 hours and 68% within 24 hours. Moreover, all DSV remained alive within 75 hours in rearing experiment. The survival rates of EUV was 56% just after thawing and then it was gradually reduced to 34% within 24 hours. All EUV larvae remained alive until 57 hours. All stages of thawed larvae could not developed to next stage. The LAI by the elapsed time in post-thawed larvae was shown as Fig 6. The initial LAI of TCP was 3.4 and it decreased as time passed. It was 2.6 after 12 hours and no movement was observed after 24 hours. On the other hand, the initial LAI of DSV was 3.4 and it decreased to 2.3 after 48 hours and 1.1 after 72 hours. The thawed DSV larvae showed no movement at 75 hours. LAI in EUV also showed the similar tendency to that in DSV. Eventually, it was reached to 0 after 57 hours.
DISCUSSION
Selection and appropriate concentration of CPA, equilibrium time, and freezing rate, are important factors which is necessary for effective cryopreservation as well as subsequent survival at different developmental stage.
The developmental stage that has been used in cryopreservation studies of shellfish developing embryos is mostly in early mitotic stage. Toledo et al. (1989) reported the cryopreserving possibility on 2- to 8-cell stages and trochophore of development in blue mussel.
Gwo (1995) stated that among different developmental stages of oyster including morula, gastrula and trochophore, trochophore found to have higher tolerance to CPA than morula or gastrula. Chao et al. (1994) also found that early stage embryos were more vulnerable to higher consentration CPA than late stage embryos. In the present study, we obtained the similar results that late stage larva had higher tolerance to CPA than early stage larvae among TCP, DSV and EUV of oyster.
The production of seedstock from cryopreserved larvae is important because presently maintenance of broodstock requires considerable space and expense within a hatchery or entails the risk of placing valuable stocks into natural waters where they are susceptible to diseases, predators and contamination with wild spat fall (Paniagua-Chavez et al., 1998).
The improvement of cryopreservation technique had been obtained for spat production. However, most of those research only concentrates on oyster species, especially trochophore and unfertilized egg: Eastern oyster Crassostrea virginica (Paniagua-Chavez et al., 1998) and Pacific oyster Crassostrea gigas (Usuki et al., 2002; Tervit et al., 2005). Since the seed production had been successfully researched, there was no report on cryopreservation during last 15 years. Taking into account these important facts, we need to further study the problems linked with cryopreservation research.
According to rearing time on survival rates of TCP, larvae could not remain alive until 24 hours, DSV until 75 hours and EUV until 57 hours. It can be inferred from these observations that structural damage leads to abnormality in physiological metabolism and to death, eventually.
In the present study, best survival rate was recorded, when 0.2 M sucrose and 2.0 M EG were used to cryopreserve oyster larvae and the freezing rate for TCP, DSV, and EUV was set at –1°C/min. These results confirmed that these CPA concentrations and freezing rate are the ideal conditions for cryopreservation of oyster larvae. Even though cilia movement after freezing and thawing was active, there was reduction in survival rate with the increase of larval rearing time and this seems to be because not enough CPA entered cytoplasm and there were some damage occurred to the cell due to crystal when being frozen. For successful cryopreservation of spat production, appropriate type, concentration, permeation time of CPA and freezing method are needed to be explored step-wisely.