INTRODUCTION
The fish family Gobiidae are known as about 1,975 species in 275 genera around the world (Nelson et al., 2016), and about 76 species in 39 genera are reported in Korea (NIBR, 2017). Luciogobius grandis is a fish belonging to the family Perciformes Gobiidae, and is known to be distributed in the coastal and intertidal zone of the East and South Seas of Korea. (Kim et al., 2005).
The characteristics of osteological development of larvae is essential to detect and eliminate skeletal abnormalities occurring in early breeding in the process of seed production (Koumoundouros et al., 1997a, b), and, providing systematic basic traits of the young stage, the researches in this area are active (Mook, 1977; Potthoff et al., 1987, 1988; Potthoff & Tellock, 1993; Liu, 2001; Sfakianakis et al., 2004; Ҫoban et al., 2009).
The studies on L. grandis have done with the egg development and the larvae morphology development (Yun et al., 2008), and the studies on the skeleton of the fish family Gobiidae are Chaenogobius laevis (Kim & Han, 1989), Periophthalmus cantonensis (Lee, 1990), L. guttatus (Kim et al., 1992), Tridentiger trigonocephalus (Han et al., 2018), T. obscurus (Hwang et al., 2018). The fish family Gobiidae is difficult to classify its species because there are species that have the same habitats or are similar externally. L. grandis is very similar externally to L. guttatus and the taxonomic studies are needed to identify differences between the two species. Therefore, this study is intended to be used as the basic material for the taxonomic research, as observing the stages of skeletal development of L. grandis larvae by the stages of growth, and comparing and examining the skeletal development patterns of the same fish family of Gobiidae.
MATERIALS AND METHODS
The samples used in the study were collected from individuals showing allomothering and transported to the laboratory at the spawning site for spawned eggs and species identification under a rock in a small river connected to the sea located in Ocheon-dong, Yeosu-si, Jeollanam-do Korea in May 2006. It followed Kim et al. (2005) for the species identification.
The fertilized eggs attached to the rock was housed in a square tank (45×30×20 cm), and was gently aerated by aeration after medicine bathing with 5% neutral formalin. It was maintained the breeding water temperature at 18°C–21°C (average 19.5±1.5°C), and salinity at 30–33 psu (average 31.5±1.5 psu), from the salt concentration (YSI 556) was measured using a water quality meter. 21 days after hatching (DAH), it was fed Brachionus rotundiformis to the hatched larvae after completing yolk absorption, and fed 1–2 Artemia sp. nauplius per mL. From 42 DAH, feeding was carried out while mixing and feeding the microdiet particles (Dry feed, Jeilfeed, haman, Korea).
In order to observe the skeletal development of larvae, 5 specimens were collected at intervals of 3 to 10 days from just immediately after hatching to 51 days and measured their sizes to 0.01 mm with a profile projector (V-12B, Nikon, Tokyo, Japan), and, after fixing them in 5% neutral formalin, the tibia was stained according to the staining method of Kawamura & Hosoya (1991). The shape of the skeleton was sketched after observing with a anatomy microscope (NM-40, Nikon), and the name of each part of the skeleton was according to Kim et al. (1992).
RESULTS
The head was a flat shape with a opercle, the dorsal fin was located at the back from the center, and the caudal fin edges were rounded. The meristic characters were 16 dorsal fins and 16 anal fins, and the lateral line scales were not observed. In the pectoral fin, two or more separated fin-rays appeared, showing a difference from the similar species, Luciogobius guttatus identified as L. grandis (Kim et al., 2005).
The skeleton of L. grandis larvae was divided into cranial, shoulder girdle, vertebrae, and coccyx, and the development process of the growth stage was shown in Figs. 1, 2; Tables 1–3. On the 3 DAH, the preflexion larvae was 3.86–4.13 mm in total length (TL) (average 4.01±0.11 mm, n=5), and the frontal began to ossify in the skull, and the parasphenoid ossified across the eyeball. The premaxillary and maxillary of the upper jaw were ossified in the jaw bones with intake function and the dentary was ossified in the lower jaw. The clavicle was ossified in the shoulder girdle bone (Fig. 1A).
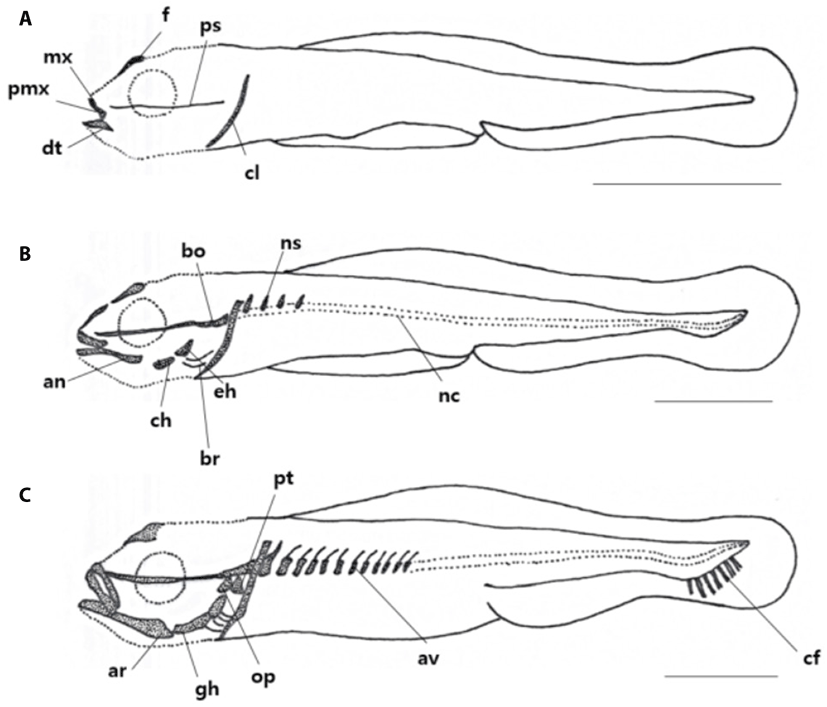
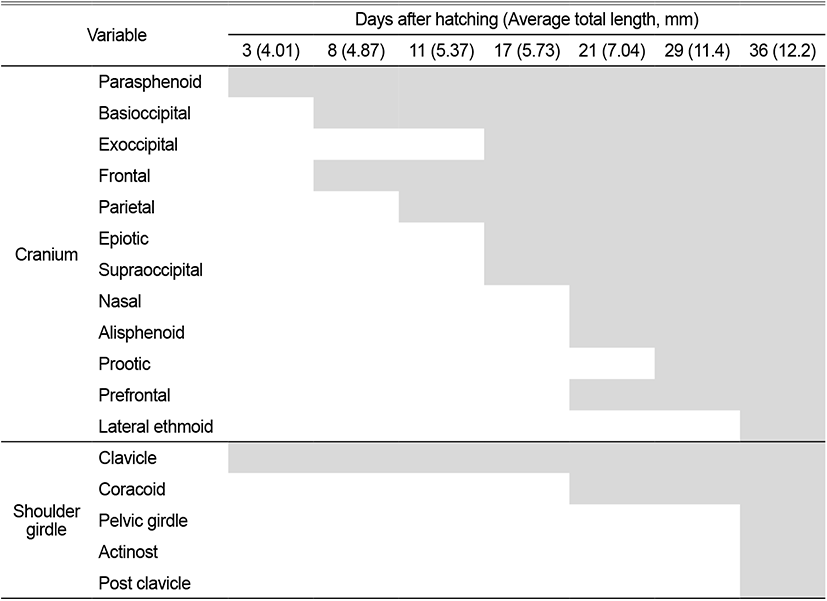
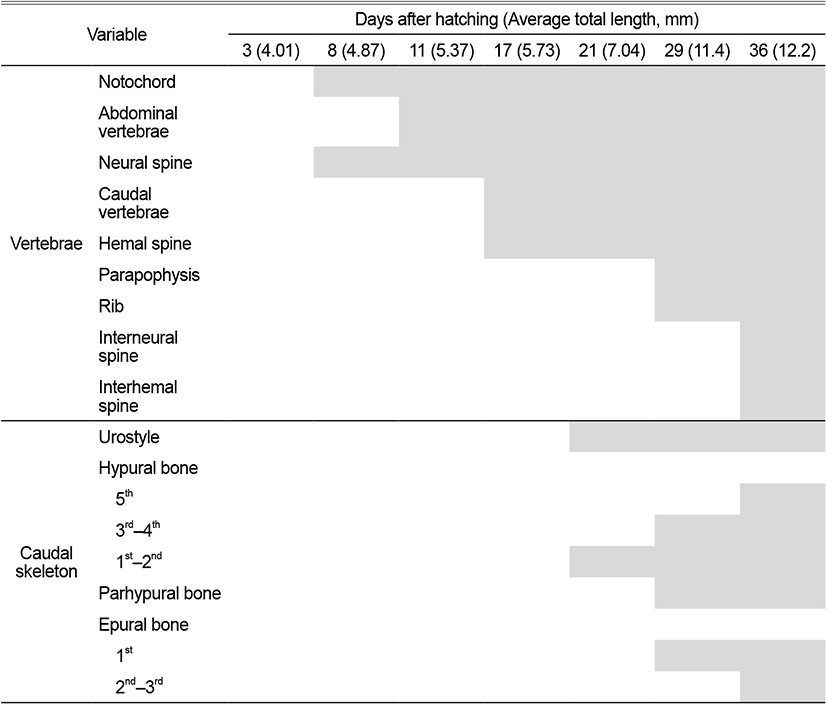
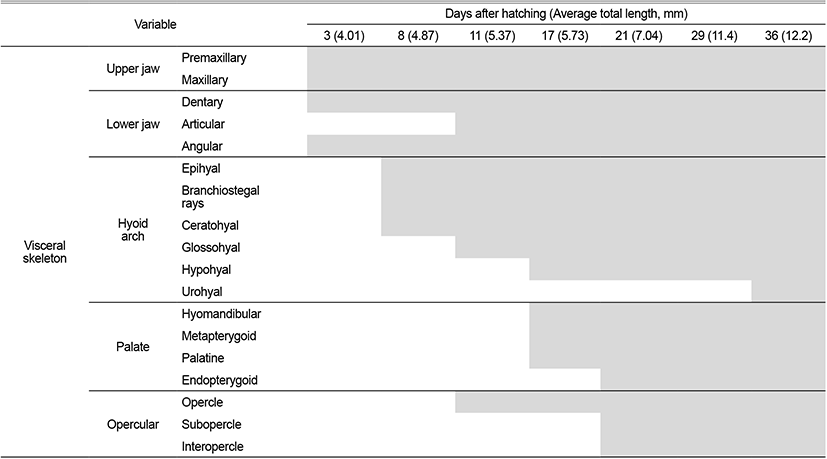
On the 8 DAH, the preflexion larvae was 4.77–4.95 mm in TL (average 4.01±0.11 mm, n=5), the basioccipital was ossified in the cranial bone, the angular was ossified in the palate and set in the dentary bone of the lower jaw. The epihyal and ceratohyal were ossified in the palatoglossal arch, and two branchiostegal rays began to ossify under the upper hyoid bone. The vertebrae that forming the central axis of the body was not ossified and consisted of notochord, and the neural spine began to ossify above the abdominal vertebrae (Fig. 1B).
On the 11 DAH, the advanced postflexion larvae was 5.30–5.44 mm in TL (average 5.37±0.05 mm, n=5), the palatine was ossified in the cranial bone, the articular was ossified in the palate and set in the angular bone. The glossohyal was ossified in the palatoglossal arch, the opercle began to ossify in the branchial arch. The vertebrae which consisted of the spine began to develop as the abdominal bone ossified. The caudal fin rays at the distal end of the body began to develop (Fig. 1C).
On the 17 DAH, the advanced postflexion larvae was 5.62–5.85 mm in TL (average 5.37±0.05 mm, n=5), the supraoccipital and epiotic were ossified in the cranial bone, the hyomandibular, metapterygoid, palatine were ossified in the palate, and the hypohyal was ossified in the palatoglossal arch. In the vertebrae, the caudal vertebrae began to ossify with the hemal spine as the abdominal bone was ossified with the nerve toward the tail. (Fig. 2A).
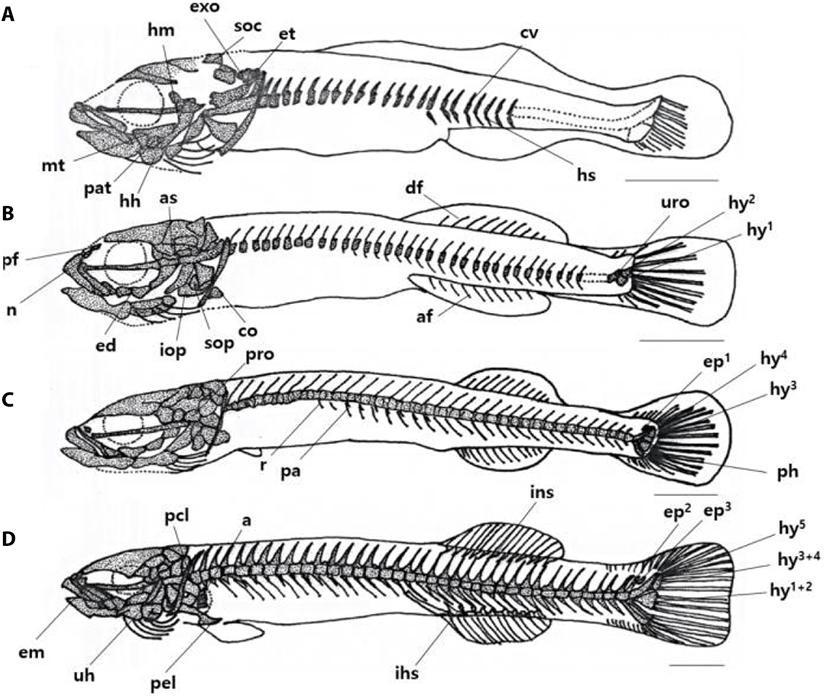
On the 21 DAH, the advanced postflexion larvae was 6.91–7.17 mm in TL (average 7.04±0.10 mm, n=5), the prefrontal, alisphenoid, and nasal were ossified in the cranial bone, the endopterygoid was ossified in the palate, and the subopercle, interopercle were ossified under the opercle in the opercular.
The coracoid was ossified in the shoulder girdle bone, and the dorsal fin rays and anal fin rays developed as the primordial fin was divided into dorsal, anal, and tail fin. In the coccyx, the vertebral end was bent upward, and the urostyle began to ossify, and at the bottom, two hypural bones ossified (Fig. 2B).
On the 29 DAH, the advanced postflexion larvae was 11.2–11.8 mm in TL (average 11.4±0.24 mm, n=5), the prootic was ossified in the cranial bone, and the rib and parapophysis were ossified under the abdominal vertebra. In the coccyx, 2 hypural bones were additionally ossified, the parhypural bone was ossified under the first hypural bone, and 1 epural bone was ossified between the neural spine and urostyle (Fig. 2C).
On the 36 DAH, the juvenile was 12.0–12.5 mm in TL (average 12.2±0.20 mm, n=5), the lateral ethmoid was ossified in the cranial bone, and the urohyal was ossified in the hyoid arch, and the pelvic girdle was ossified in the ventral.
The actinost and the post cleithrum were ossified in the shoulder girdle bone, and the interneural spine and the interhemal spine were ossified in the pterygiophore supporting the dorsal and anal fins. Two pural bones were additionally developed in the coccyx, and one hypural bone was ossified in addition, and then, the first, second, third, and fourth were combining and made of three bone fragments, and the bone ossification of all skeletons was completed (Fig. 2D).
DISCUSSION
The skeletal development of L. grandis was not observed just immediately after hatching. While growing, on the 3 DAH when the average TL was 4.01 mm, the frontal and the parasphenoid in the cranial bone began to ossify. In this period, as the yolk absorption was completed, the feeding function developed and the premaxillary, maxillary, and dentary were ossified. In addition, swimming ability developed as the clavicle of the shoulder girdle bone ossified. The skeletal development of T. trigonocephalus (Han et al., 2018), the same fish family Gobiidae, was began when the average TL was 4.44 mm on 7 DAH. The skeletal developments were began when L. guttatus (Kim et al., 1992) was 5.50 mm in TL on 11 DAH, when C. laevis (Kim & Han, 1989) was 6.00 mm in TL on 9 DAH, and when T. obscurus (Hwang et al., 2018) was 3.62 mm in TL on 8 DAH.
The fish family Gobiidae has an egg yolk right after hatching, so it survives for a period of time without feeding, during this period, the mouth opens for feeding and the swimming ability develops, and it is known that the development of feeding function and the role of clavicle are related (Wagemans & Vandewalle, 1999).
The shoulder girdle bone of L. grandis developed in the order of clavicle, coracoid, actinost, and post cleithrum when the average TL was 4.01 mm on 3 DAH, T. trigonocephalus (Han et al., 2018), a fish family Gobiidae, was ossified in the order of clavicle, coracoid, and actinost when the average TL of 4.44 mm on 7 DAH, and L. guttatus (Kim et al., 1992) ossified in the order of clavicle, coracoid, scapula, and actinost when the average TL was 5.50 mm on the 11 DAH. C. laevis (Kim & Han, 1989) ossified in the order of clavicle, scapula, and actinost when the average TL was 6.30 mm on 13 DAH, T. obscurus (Hwang et al., 2018) ossified in the order of clavicle, actinost, coracoid, and scapula on 13 DAH, as it showed a slight difference according to the order of ossification, but it was a relatively similar developmental pattern.
L. grandis was observed to have one pore formed in the scapula when the average TL was 12.2 mm on 36 DAH, mostly observed in the same fish family Gobiidae, and the formating pores in the scapula is known as a taxonomic trait that appears only in L. japonicus, but is considered a general trait observed in various fish (Han et al., 2018).
Specifically, the number of vertebrae has been used as a taxonomical character (Yamada et al., 2009). Table 4 compares the number of vertebrae in fish of the genus Luciogobius. The vertebrae development of L. grandis began to abdominal vertebrae ossification from head to tail when it was 4.87 mm on the 8 DAH, after the neural spine ossified, and when the average TL is 5.37 mm on 11 DAH. T. trigonocephalus (Han et al., 2018), the same fish family Gobiidae, began to ossify abdominal vertebrae at an average TL of 5.02 mm on 10 DAH, and when L. guttatus had an average TL of 6.10 mm on 16 DAH, the neural spine was ossified simultaneously with the ossification of the abdominal vertebrae. In C. laevis (Kim & Han, 1989), the abdominal vertebrae and caudal vertebrae ossified simultaneously at an average TL of 6.00 mm on 9 DAH. In T. obscurus (Hwang et al., 2018), the neural spine ossified after abdominal vertebrae ossification at 3.96 mm on the 10 DAH. L. grandis developed abdominal vertebrae after neural spine development, L. guttatus, T. trigonocephalus, and T. obscurus developed abdominal vertebrae and ossified neural spine, and hemal spine, and in C. laevis, abdominal vertebrae and caudal vertebrae were ossified at the same time, showing a difference from the order of development of L. grandis.
The stems of dorsal fin and anal fin of L. grandis developed simultaneously when the average TL was 7.04 mm on 21 DAH, T. trigonocephalus (Han et al., 2018), the same fish family, began to develop the dorsal fin stem after the posterior dorsal fin development when the average TL is 6.40 mm on 16 DAH, Mugilogobius abei (Kim & Han, 1991) was when an average TL was 3.20 mm on 12 DAH, and T. obscurus (Hwang et al., 2018) began to develop stems on the dorsal and posterior fins on the back when the average TL was 7.99 mm on 28 DAH. C. laevis (Kim & Han, 1989) began to develop the stem of dorsal fin and anal fin when the average TL was 7.05 mm on 17 DAH, and L. guttatus (Kim et al., 1992) began to develop the stem of dorsal fin and anal fin with an average TL of 6.10 mm on 16 DAH. L. grandis had the same structure as L. guttatus with one dorsal and anal fin respectively, and the development patterns of them were similar.
L. grandis developed the fin stem at the latest among the same fish family, and the same fish families, T. trigonocephalus, M. abei, C. laevis, and T. obscurus, showed differences from the fin development process of L. grandis because the dorsal fin was divided into the front and back. In addition, the dorsal fin developed from the rear to the front and was similar to the general development pattern of L. japonicus (Johnson, 1984; Faustino & Power, 1999).
The pterygiophore development of L. grandis occurred after centrum development began, and this developmental pattern appeared in the same fish family Gobiidae, and the pterygiophore was fully formed after completed the development of fin stem. The developing pterygiophore when the development of vertebrae and fins has been completed appears to be related to increased propulsion in fish swimming (Lee et al., 2001).
The coccyx of L. grandis consisted of the urostyle, the pural bone, the hypural bone, and the parhypural bone, the urostyle began to ossify when the average TL was 7.04 mm on the 21 DAH, it began to ossify that T. trigonocephalus (Han et al., 2018) was when the average TL was 9.32 mm on the 28 DAH, L. guttatus (Kim et al., 1992) was when the average TL was 7.60 mm on the 25 DAH, C. laevis (Kim & Han, 1989) was when the average TL was 7.25 mm on the 20 DAH, and T. obscurus (Hwang et al., 2018) was when the average TL was 7.99 mm on the 28 DAH, and L. grandis and T. trigonocephalus were similar in the period of urostyle and their TL.
The fusion of the hypural bone formed 3 hypural bones (1+2, 3+4, 5) when the average TL was 12.2 mm on the 36 DAH. It showed the differences as T. trigonocephalus (Han et al., 2018) formed three hypural bones (1+2, 3+4, 5), and C. laevis (Kim & Han, 1989) and L. guttatus (Kim et al., 1992) formed two hypural bones (1+2, 3+4+5).
Thus, as the shape of hypural bone appeared diverse in the same fish family Gobiidae, it is considered to be significant as the basic data for systematic research. The differences in the skeletal development of the larvae of L. grandis and L. guttatus are that L. grandis showed the faster ossification of the first skeleton, and the abdominal vertebra of vertebra L. grandis ossified after ossification of the neural spine, but, in L. guttatus, vertebrae and neural spine were ossified simultaneously and showed the difference. It could be verified the two similar species have exomorphic difference through the results of this study as L. guttatus was fused into two bone fragments, while the hypural bone of L. grandis was fused into three. In the future, the study of the skeletal development of larvae is thought to be used as a very significant basic data for understanding species identification and skeletal characteristics of an adult fish, and it seems that more researches have to be carried out to characterize the classification and skeletal characteristics of the fish family Gobiidae.

