INTRODUCTION
Many different species of herpesviruses can infect and cause diseases in mammals, birds, amphibians, reptiles, and fish (Roizman & Pellet, 2001; Hedrick et al., 2006). According to the recent classification of the International Committee on Taxonomy of Viruses (ICTV; www.ictvonline.org), there are currently 3 families in the Herpesvirales order: Alloherpesviridae, Herpesviridae, and Malacoherpesviridae. The Alloherpesviridae has 12 species koi herpesvirus (KHV), also known as Cyprinid herpesvirus 3 (CyHV-3). This species has double strand DNA and a 295-kb genome (Aoki et al., 2007). The symptoms of infection are loss of swimming coordination, apathy, gathering at water inlets, necrosis of the gill, and sunken eyes (Hedrick et al., 2005). These infections have led to considerable economic losses (Hedrick et al., 2000).
In an initial study, researchers suggested that the gills are the major organ of virus entry in carp (Gilad et al., 2004; Dishon et al., 2005), but a more recent study demonstrated that the skin covering the body of fish is a major portal of KHV entry (Costes et al., 2009). After entry, the virus spreads to the kidney, spleen, and liver and high levels of KHV DNA can detect in these internal organs. Virus replication occurs in the gills, intestine, liver, brain, and kidneys (Pikarsky et al., 2004).
There is evidence of KHV in wild and farmed fish worldwide. In Asia, there are reports of this virus in fish cultured in Japan (Sano et al., 2004), Taiwan (Tu et al., 2004; Cheng et al., 2011), China (Dong et al., 2011), Iran (Rahmati-Holasoo et al., 2016), and Korea (Gomez et al., 2011; Cho et al., 2014). KHV can be detected by a polymerase chain reaction (PCR) assay, an enzyme-linked immunosorbent assay (ELISA) or immunohistochemical (IHC) methods (Akoi et al., 2011) in carp and koi (Sano et al., 2005; Haramoto et al., 2009; Akoi et al., 2011 & Minamoto et al., 2012). Since 2007, the World Organization of Animal Health (OIE) has designated KHV as a statutory infectious disease. Infections by this virus have adversely affected the production and trade of koi and common carp (Haenen et al., 2004; Sano et al., 2004).
Fish of all ages appear susceptible to KHV (Bretzinger et al., 1999; Sano et al., 2004), but those weighing 2.5–6 g are more susceptible those weighing 230 g under experimental conditions. The gold fish×common carp hybrid has low susceptibility to this virus, with a mortality rate of only 5% (Hedrick et al., 2005). Approaches to control and prevent KHV infections mainly rely on avoiding exposure, because a safe and effective vaccine and chemotherapy are not currently available. For this reason, some researchers have proposed breeding for increased resistance to KHV infection (Hedrick et al., 2005). Thus, there is now evidence of differential resistance to KHV among different carp hybrids (Hedrick et al., 2005).
Red common carp (C. carpio) is a well-known breed of common carp that is widely used for crossbreeding and hybridization in China (Wang et al., 2006). In this study, we report the first cross breeding of koi (C. carpio) and red common carp (C. carpio) and then compared the susceptibility of these koi hybrids to experimental injections of KHV.
MATERIALS AND METHODS
Koi (Cyprinus carpio) and red common carp (Cyprinus carpio) broodstocks without any disease were reared and used for breeding experiments at the Inland Aquaculture Research Center, National Institute of Fisheries Science (Changwon, Korea). Ovulated eggs of koi were stripped and fertilized with sperm from koi and red common carp below the Table 1. Fish were used for the virus challenge test at the age of 10 months, when they weighed 9–12 g.
| Parental combination | |||
|---|---|---|---|
| Group ratio | Koi×koi (KK) 3F:3M | Koi×red common carp (KR) 3F:6M | Red common carp×koi (RK) 6F:3M |
After a 2 week adaptation in a re-circulation tank at 23°C, the fish were used for infection trials.
The KHV strain F347 (VR-1592) was purchased from American Type Culture Collection (ATCC). The virus was incubated in common carp brain (CCB) cells. The CCB cell line was maintained at 23°C in minimum essential medium (MEM, Sigma-Aldrich, St. Louis, MO, USA) and supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich), 2 mM L-glutamine, 100 IU mL−1 penicillin, and 100 μg mL−1 streptomycin (Sigma-Aldrich) for 14 days. The presence of CyHV-3 was evaluated by the presence of characteristic cytopathic effects and was confirmed by PCR (OIE, 2013). After titration, the virus was adjusted to a final concentration of 104 50% tissue culture infectious dose (TCID50) per mL−1 for intraperitoneal injection. The experiments were performed in triplicate aquaria with 4 groups (n=50): KK (koi×koi), KR (koi×red common carp), and RK (red common carp×red common carp), and uninfected fish (control). There were 150 fish per group (n=50/tank) and total 600 fish used in this study. Fish were infected by intraperitoneal injection, and kept at 23°C, and the control group was treated with saline. Fish were fed on a pellet diet and fish were observed daily for signs of disease and mortality for 45 days. Moribund fish were euthanized with an overdose of MS-222 and at the end of the 45 day period, all surviving fish were euthanized with an overdose of MS-222. Gill and kidney were sampled at 6 days and 9 days post injection, and died fish from each group until the end of the experiment. All tests were performed in gill and kidney (viral detection by PCR, histology analysis).
For histopathology and immunohistochemistry (IHC), kidneys and gills were collected (n=5) from each group on days 6 and days 9, and fixed in 4% neutral formalin for 24 h. Paraffin-embedded tissues were stained with hematoxylin and eosin (H&E) for histological observation, and analyzed by IHC staining. For IHC, heat-induced antigen retrieval was performed using 0.01 M sodium citrate buffer in a microwave for 15 min. After quenching of endogenous peroxidase and blocking in 3% goat serum, tissue was incubated with the primary monoclonal anti-KHV antibody (P14, Aquatic Diagnostic). After three washes with Tris-buffered saline (TBST), followed by addition of the secondary antibody (goat anti-mouse IgG) peroxidase activity was detected with 32-diaminobenzidine-tetrahydrochloride (DAB, Abcam). To investigate the specificity of these reactions, isotype control as negative control and a known infected individual as positive control were used. Finally, the samples were examined under optical microscopy (AxioCam MR, Carl Zeiss, Oberkochen, Germany).
KHV DNA was detected using the CyHV-3-specific PCR (OIE, 2013). DNA was extracted from gills and kidneys using Qiagen DNeasy Blood & Tissue kit, and PCR amplification was performed using specific primers (Table 2). The PCR products were subjected to electrophoresis in an agarose gel (1%), visualized by UV transillumination, and photographed.
| Primer | Sequence | Product size (bp) | Reference |
|---|---|---|---|
| TK F | 5’-GGGTTACCTGTACGAG-3’ | 409 | OIE (2013) |
| TK R | 5’-CACCCAGTAGATTATGC-3’ | ||
| SphI-5 F | 5’-GACACCACATCTGCAAGGAG-3’ | 292 | OIE (2013) |
| SphI-5 R | 5’-GACACATGTTACAATGGTCGC-3’ |
RESULTS
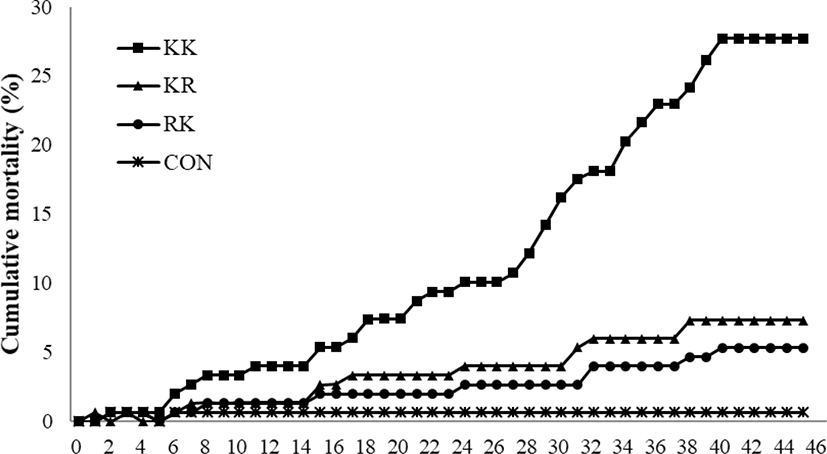
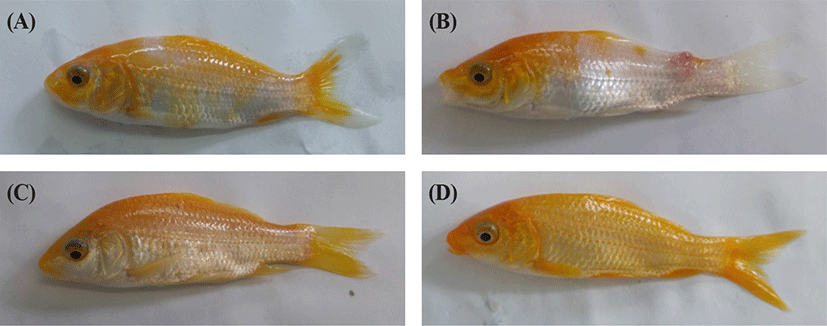
Fig. 1 shows the cumulative mortality from the KHV challenge-test. We first detected diseased fish at 3 days post infection (p.i.), which were floating on the water surface in the culture tank. Mortality started between day 5 and day 7 p.i. depending on the group. The mortality of the uninfected controls was 0% throughout the experiment, but the infected KK group reached 28% by day 40 p.i. The cumulative mortality was 8% in the KR group and 6% in the RK group. Previous study observed that the KHV infected juvenile red common carp had 100% survival by day 50 p.i. but not juvenile koi (data not shown). Diseased fish had hemorrhages, mucus production, and necrosis in gills, and these were more severe in the KK than in the RK and KR (Fig. 2). We observed no abnormalities on the body surface and gills of RK fish (Fig. 2).
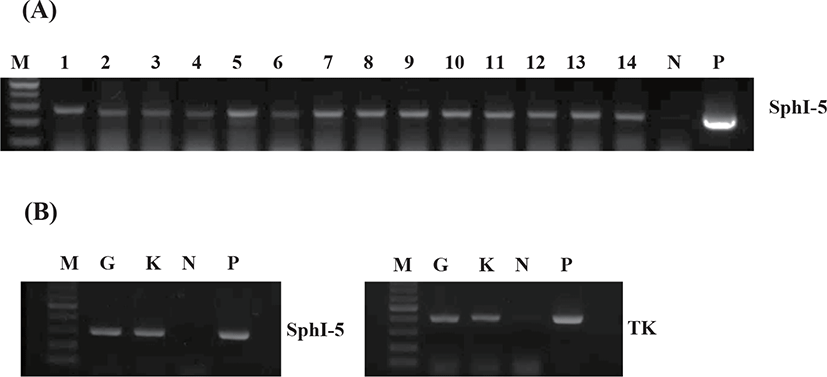
Our PCR experiments indicated the presence of KHV DNA in all dead fish (Fig. 3A). In particular, a KHV-specific DNA (TK and SphI-5) was detected in the gills and kidneys of dead fish (n=3) from KK group (Fig. 3B).
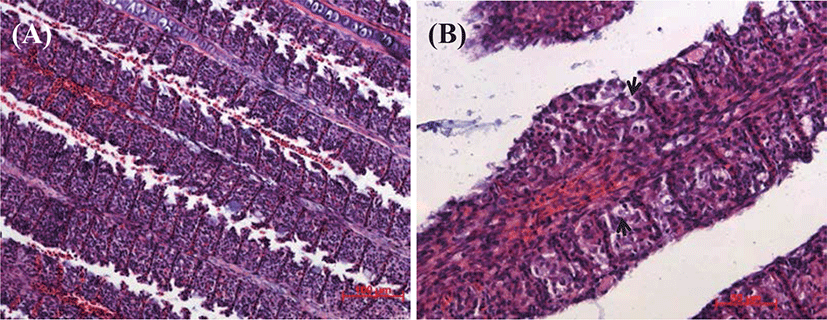
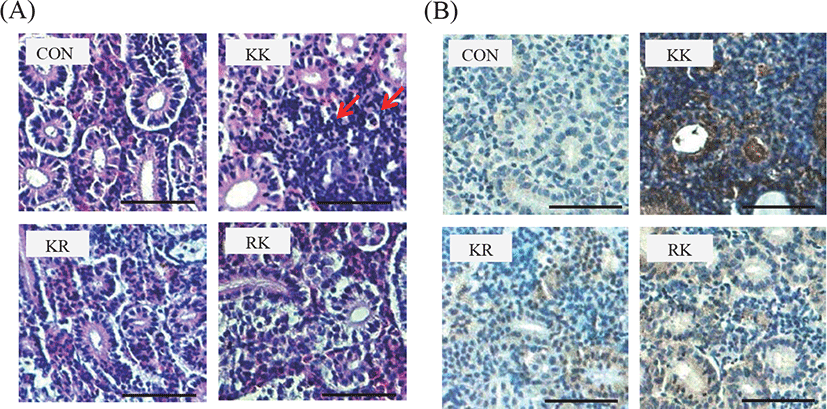
There were also histopathological changes in the gills of infected fish (Fig. 4). In KK fish infected KHV, fusion in gill lamellae with swollen were observed and the gill showed necrosis of the epithelial cells on day 9 (Fig. 4B). Examination of sectioned and stained kidney from KHV-infected KK revealed severe infiltration of lymphocytic-cells at day 6 p.i., with necrosis of the renal tubules and especially severe pathology (Fig. 5A). IHC analysis revealed the KHV antigen was present in the kidney (Fig. 5B). KHV proteins were detected at high levels in the KK group, whereas they were detected at low level in the KR and RK group. The KHV protein was more abundant in the cytoplasm than the nuclei of the large polygonal cells infiltrating the renal interstitium. There was no reactivity in the tissues of uninfected control fish (Fig. 5B).
DISCUSSION
Previous researchers proposed cross-breeding as a potential method to improve fish resistance to KHV disease (Shapira et al., 2005; Hedrick et al., 2006), and studies of the genetics of disease resistance in fish support this strategy (Chevassus & Dorson 1990). Previous studies have established hybrid cultured carps (Bergmann et al., 2010b). In particular, Israeli carp strains (Dor-70 and Nasice) that were crossbred with a native Czech strain (Sasson) had significantly higher survival rates following KHV infection (64% and 69%) than either parental strain (Dor-70: 28%; Nasice: 9%) (Shapira et al., 2005). Similar experiments in the UK showed that crossbreeding carp with a ‘wild’ strain originating from the Amor or Duna Rivers in Hungary produced carp that were more resistant to KHV than domesticated carp (Dixon et al., 2009). Other studies examined koi×crucian carp (Carassius carassius) hybrids and koi×goldfish hybrids, and reported that both hybrids showed signs of KHV. Even though the koi×crucian carp hybrid had similar mortality levels as pure carp following KHV-I infection (91% and 100%, respectively), the koi×goldfish hybrid had reduced mortality (35%; Bergmann et al., 2010b). Little is known about the resistance of koi and red common carp cross. In the current study, we crossbred koi and red common carp and evaluated their resistance to KHV. The results show that all tested hybrid fish had improved resistance to KHV relative to KK fish, but the hybrids also had some susceptibility to KHV.
Our results are inconsistent with the findings of Bergman et al. (2009) and Hedrick et al. (2006), who demonstrated high susceptibility of koi×goldfish hybrids (35% to KHV-I and 42% to KHV-E). Our results demonstrated a higher survival rate by RK cross (94%) and KR cross (92%) than KK fish (72%) at 40 days after intraperitoneal injection of the KHV virus. The CyHV-3 can be subdivided in 3 main genotypes: one from Japan (KHV-J), one from Israel (KHV-I), and one from the United States and Europe (KHV-U). In this study, we purchased viral DNA from the UK (VR-1592). The survival rate in our experiments (KK: 72%) may differ from that reported by other groups (koi and carp: 10%–30%) due to the use of different KHV genotypes, different infection methods, or different fish strains or breeding methods. Other studies of virus infections of carp— pure lines or hybrids—indicated the presence of different genetic sources of resistance due to the large range of survival rates (0%–98%) (Dixon et al., 2009; Rakus et al., 2009; Shapira et al., 2005)
The presence of CyHV-3 can be verified by amplification of the TK gene using PCR (Bercovier et al., 2005), an ELISA (Adkison et al., 2005), real-time PCR, or nested-PCR (Bergmann et al., 2010a). We performed PCR to confirm the presence of CyHV-3 (OIE, 2013), and also performed IHC in gill and kidney tissues. We found viral DNA in all dead fish, and our IHC assay detected the KHV antigen in the kidneys of all infected fish. The presence this antigen is a reliable indicator of exposure to the virus. And also, the CyHV-3 was re-isolated from the kidney of infected fish. The CyHV-3 virus confirmed by the presence of CPE and was detected by PCR on day 5 (data not shown).
The major histocompatibility complex (MHC) is an important component of disease resistance. Many studies have established an association between MHC diversity and resistance to pathogens (Rakus et al., 2009; Li et al., 2011; Zhao et al., 2012). In common carp, MHC class II B genes may be markers for resistance to CyHV-3 (Rakus et al., 2009), and in purse red common carp (Cyprinus carpio), MHC class II α alleles may be associated with resistance to Aeromonas hydrophila (Liu et al., 2014). However, the mechanism of KHV resistance in RK and KR hybrids is unknown. In our previous study, we reported that Interleukin (IL)-12 p35 and Toll-like receptor (TLR) 9 were significantly high expressed on 48 h p.i in RK (Hwang et al., 2017) and our future studies will seek to identify candidate genes for KHV resistance in these cross groups using 2-dimensional electrophoresis and proteomics.
In conclusion, our results indicate that two koi cross -KR and -RK were more resistant KHV than KK fish. Use of such cross may therefore be a possible approach to reduce serious mortality due to KHV infection.

