INTRODUCTION
Melanoma is a type of skin cancer that develops from malignant transformation of melanocytes. While it is less common than other skin cancers such as basal cell carcinoma and squamous cell carcinoma, melanoma is the deadliest one of skin cancers due to its aggressive spreading ability to other organs if not treated at its early stage. Over the last decade, the annual cases of melanoma increased by nearly 50%, and International Agency for Research on Cancer (IARC) reported that approximately 287,000 new cases were diagnosed globally in 2018 (Ferlay et al., 2019). Although surgical resection of tumor mass is often curative in the early stage of melanoma progression, melanoma remains one of the cancers resistant to systemic therapy with 5-year survival rate of 25% for patients with distant metastasis (American Cancer Society, 2020). However, recent major advancements in molecular targeted and immune-based therapies have made remarkable progress for the treatment of this disease (Domingues et al., 2018).
Comprehensive genetic analysis of cancers have revealed that melanoma has the most frequent mutation rate of all cancers (Alexandrov et al., 2013). In most melanomas, the mitogen-activated protein kinase (MAPK) signaling pathway (also known as RAS/RAF/MEK/ERK pathway) is commonly active through mutations in BRAF (40%–60%) and NRAS (15%–25%), which eventually lead to uncontrolled cell growth, proliferation and survival (Leonardi et al., 2018). These somatic mutations in melanoma have become principal therapeutic targets and then led to the development of novel targeted drugs, such as inhibitors of BRAF (dabrafenib, vemurafenib and encorafenib) and MEK (trametinib, cobimetinib and binimetinib) (Smalley, 2010). Currently, oncologists typically used the combination of BRAF and MEK inhibitor for the treatment of metastatic BRAF-mutant melanoma (Goyal & Silberstein, 2015). Despite remarkable advances for the treatment of metastatic melanoma, however, there are significant therapeutic challenges due to acquired resistance or intrinsic unresponsiveness to targeted agents (Holderfield et al., 2014; Long et al., 2014). Moreover, recent studies have reported that MEK inhibition enhances metastatic properties such as cell invasion and migration, which could result in the exasperation of malignancy and subsequent treatment failure (Vultur et al., 2014; Zhao et al., 2017; Lee & Park, 2021).
Metformin is an oral biguanide agent and has been used as first-line medication for type II diabetes mellitus for a period of over 60 years. It lowers blood glucose levels by reducing hepatic gluconeogenesis and skeletal muscle glucose uptake (Shaw et al., 2005). In addition to its glucose-lowering effect, retrospective epidemiological studies have showed that administration of metformin at therapeutic dose reduced the risk of cancer incidence, compared to other antidiabetic medications in diabetic patients (Evans et al., 2005; Bowker et al., 2006). Thereafter, substantial amount of preclinical studies have revealed the anticancer activity of metformin in various types of cancer (Vancura et al., 2018). Several in vivo and in vitro studies by using melanoma cell lines suggested that single agent metformin or combination with BRAF/MEK inhibitors suppress melanoma cell survival and mouse xenograft growth (Niehr et al., 2011; Vujic et al., 2015). Metformin induces cell cycle arrest and apoptosis by interfering with PI3/KmTOR (phosphoinositide 3-kinase/mammalian target of rapamycin) pathway, a parallel signaling pathway of the MAPK pathway (Woodard & Platanias, 2010). It also reported to inhibit melanoma cell invasion and metastasis development by suppressing the expression of factors involved in epithelial-mesenchymal transition (EMT) (Cerezo et al., 2013; Tseng et al., 2019).
The present study undertook to investigate whether metformin can potentiate the anticancer effect of MEK inhibitor binimetinib by using melanoma cell line. In addition, our previous report using A375 melanoma cell line showed that MEK inhibitor trametinib increases the expression of EMT regulators and melanoma cell motility, which are suppressed by addition of metformin (Lee & Park, 2021). A recent report, however, documented that another MEK inhibitor binimetinib inhibited migration capacity of melanoma cells (Ryabaya et al., 2019). In this study, we try to elucidate these differential effects of binimetinib on cell migration further by using G361 melanoma cell. Here we demonstrate that metformin synergistically potentiates the growth inhibitory effect of binimetinib. The present study also shows that binimetinib increases the expression of EMT regulators and enhances cell motility, and metformin counteracts the binimetinib-induced increase of cell motility. These results suggest that metformin with binimetinib might be useful as a potential therapeutic agent against cell survival and metastatic activity in melanoma patients.
MATERIALS AND METHODS
We purchased the human melanoma cell line G361 from Korean Cell Line Bank (Seoul, Korea). The cells were cultured in RPMI 1640 (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% (v/v) heat inactivated fetal bovine serum (Gibco BRL, Bethesda, MD, USA) and 1% streptomycin/penicillin at 37℃ in a humidified atmosphere consisting of 5% CO2 and 95% air. Cells were maintained mycoplasma free by treating 5 μg/mL of Plasmocin (InvivoGen, San Diego, CA, USA). Binietinib (LC Laboratories, Woburn, MA, USA) was initially dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) to a concentration of 1 mM and further diluted in RPMI 1640 media. Metformin (also known as 1,1-dimethylbiguanide hydrochloride) was purchased from Sigma-Aldrich and dissolved in RPMI 1640 media to a working concentration of 100 mM. The final concentration of DMSO in the culture media did not exceed 0.1% (v/v).
MTT assay was performed to measure cell viability as described previously (Ko et al., 2020). Briefly, cells were harvested and seeded in 24-well plates at a concentration of 5×104 cells/well for 24 h. Then, cells were treated with increasing concentrations of binimetinib (20–80 nM), metformin (0.5–2 mM), their combinations or vehicle control for 72 h. Experiments were performed in triplicate, each conducted in quadruplicate. The IC50 values (concentrations of drugs resulting in 50% decrease in cell viability relative to controls), combination index (CI) and drug reduction index (DRI) were calculated using CompuSyn software (ComboSyn, Paramus, NJ, USA). The CI value is a quantitative measure of the degree of drugs interaction. According to the recommendation of Chou-Talalay (Chou & Talalay, 1981), CI<1 indicates synergistic effects of drugs; CI=1 indicates additive effect; CI>1 indicates antagonism.
Western blotting assays were carried out as previously described (Ko et al., 2020). Primary antibodies included ERK, pERK, SPARC, integrin αV, fibronectin, N-cadherin, Slug, β-actin and GAPDH (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following incubation with secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology, Danvers, MA, USA), immunoreactivity was detected with enhanced chemiluminescence method (Santa Cruz Biotechnology).
Cells were plated in 6-well culture dishes at a density of 600 cells per well. After 24 h, cells were treated with metformin, trametinib and their combination. Every three days, medium was changed with fresh medium containing the corresponding concentration of the drugs. Following 12-day treatment, cell colonies were washed with cold PBS and then fixed with ice-cold 100% methanol. Cells were stained with 0.1% crystal violet in 20% methanol for 10 min and pictures were taken with a digital camera (Olympus Korea, Seoul, Korea).
G361 melanoma cells were grown to 80% confluence in 12-well culture plates in complete medium. After serum-starving cells for 24 h, a 200-μL tip was used to create a consistent scratch in the cell monolayer. All wells were then carefully washed with culture medium and drug solutions in complete medium were added and incubated for 24 or 48 h. Photographs were taken with an Olympus 1×70 microscope and an Olympus DP72 camera and DPController Software (Olympus Korea).
RESULTS
To assess the response of G361 melanoma cells to treatment with binimetinib, metformin and their combination, we first carried out MTT cell viability assays. G361 cells were treated with varying concentrations of drugs as single agent or their combination for 72 h. As shown Fig. 1A, relative cell viability was reduced following treatment with binimetinib and metformin in a dose-dependent manner. IC50 values (concentrations of drugs resulting in 50% decrease in cell viability relative to controls) for binimetinib and metformin were 40.3 nM and 1.53 mM, respectively.
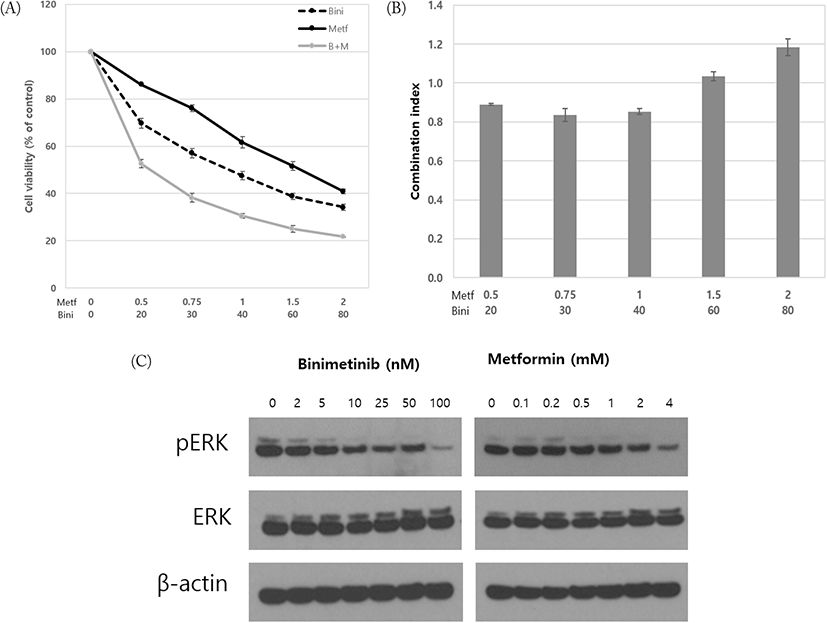
We then analyzed the growth inhibitory effect by combining metformin with binimetinib in constant ratios to each other. To quantify the effects of drug combination, we used the CompuSyn software to obtain the CI value for each combination therapy. The CI values in combination at low doses ranged from 0.89 to 1.03 indicating synergism or additivity according to the method of Chou-Talalay (Chou & Talalay, 1981). In contrast, CI value at high dose (at 2 mM of metformin and 50 nM binimetinib) was 1.18 denoting antagonistic effect between two drugs (Fig. 1B). However, CI value at ED50 (the dose effecting 50% of growth inhibition compared to control) was 0.80, showing synergism between two drugs.
We previously suggested that ERK activation in response to metformin serves as a potential predictive biomarker in the combination therapy by using metformin with other targeted agent (Lee & Park, 2021). Therefore, we performed western blotting for ERK activation following treatments with metformin and binimetinib. As shown in Fig. 1C, treatments with metformin and binimetinib suppressed the level of pERK in dose-dependent manner.
Our present cell viability assay showed that combinatory treatment of metformin with binimetinib synergistically inhibits the survival of G361 melanoma cells. To extend these cell survival results into long-term effect of the combination treatment, we next performed colony formation assay by culturing G361 melanoma cells for 12 days. As shown in Fig. 2, treatment with metformin or binimetinib as single agent led to a partial inhibition of colony formation, while the combination treatment potentiated the inhibitory effect on the formation and growth of cell colonies as compared to either agent alone. These results further confirmed the synergistic inhibitory effect of metformin with binimetinib on cell survival analyzed by using MTT assay.

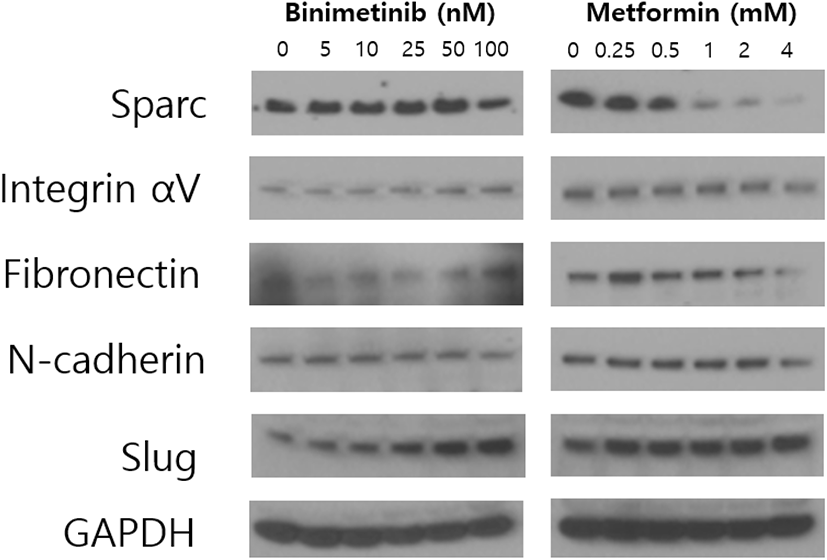
Several research groups documented that metformin suppresses cancer cell motility and metastasis development (Cerezo et al., 2013; Tseng et al., 2019). On the other hand, recent studies including us have revealed that MEK inhibition increases metastatic properties in melanoma and breast cancer cells, which could result in the deterioration of malignancy and treatment failure (Vultur et al., 2014; Zhao et al., 2017; Lee & Park, 2021). To confirm this issue further, we performed western blot assay using G361 melanoma cells to determine whether metformin and binimetinib affect the expression of factors involved in EMT. As shown in Fig. 3, treatment with binimetinib increased the expression level of integrin αV, fibronectin and slug, and exerted little effect on the expression of sparc and N-cadherin. In contrast, metformin remarkably decreased the levels of sparc, integrin αV, fibronectin and N-cadherin in dose-dependent manner.
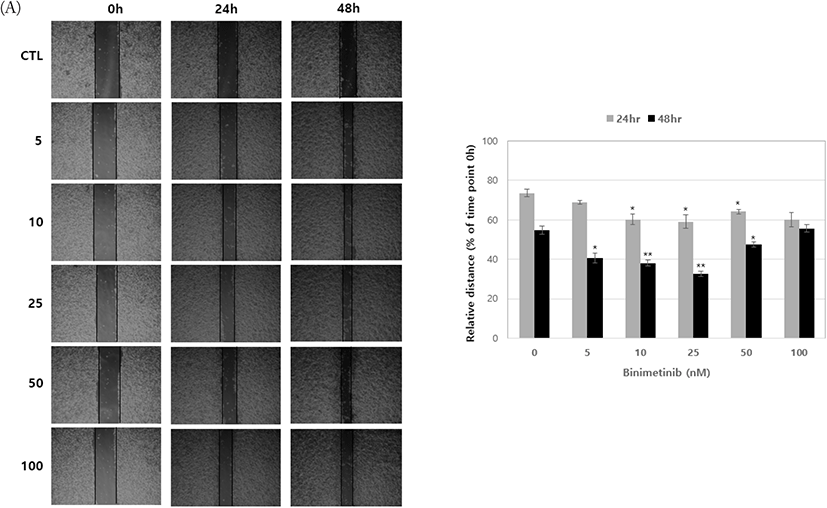
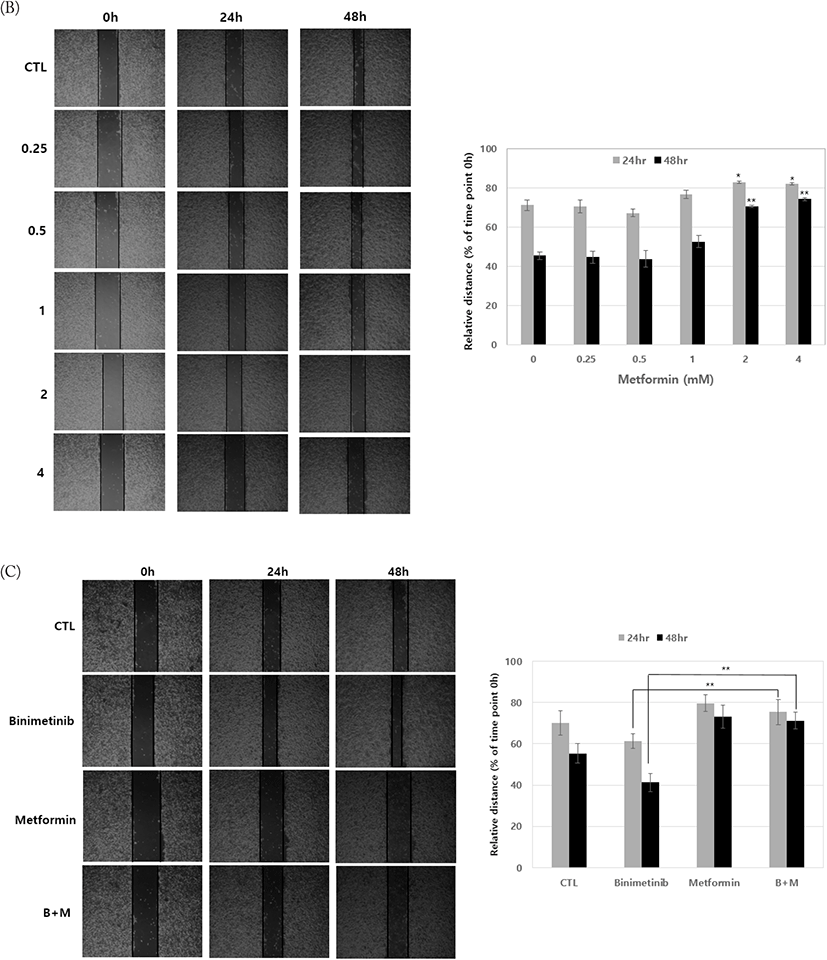
Next, we wished to determine whether the molecular changes found in the expression of EMT regulators following treatment with metformin and binimetinib lead to functional alteration, such as cell motility. For this purpose, we monitored cell migration in G361 cells using wound healing assay. Low doses of binimetinib (5–50 nM) promoted cell migration but showed little effect at high dose (100 nM) (Fig. 4A). In contrast, treatment with low doses of metformin (0.25–1 mM) alone neither enhanced nor inhibited cell migration compared to control, but high doses of metformin (2–4 mM) inhibited cell migration (Fig. 4B). Combined treatments of metformin with binimetinib suppressed the binimetinib-induced cell migration (Fig. 4C).
DISCUSSION
Molecular targeted therapy against cutaneous melanoma with BRAF mutation has shown a remarkable progress with the advent of small molecule inhibitors of BRAF itself or its downstream effector MEK (Smalley, 2010; Domingues et al., 2018). Combined treatment of BRAF inhibitor (dabrafenib or vemurafenib) with MEK inhibitor (trametinib) proved to display clinical benefits, such as better overall response rate and progression free survival, than each monotherapy (Long et al., 2018). However, half of patients treated with the combination therapy eventually experience fatal progression of their disease at 9–10 months following initial treatment, mainly due to drug resistance with severe toxicities (Long et al., 2014; Welsh et al., 2016). One of the mechanistic factors underlying the drug resistance arising from the combination therapy of BRAF and MEK inhibitors is the compensatory activation of parallel signaling cascades such as PI3K/mTOR pathway (Welsh et al., 2016). Another therapeutically important problem is that MEK inhibition reported to enhance metastatic activities such as cell migration and invasion in melanoma and breast cancer cells (Vultur et al., 2014; Zhao et al., 2017). Thus, there remains an unmet urgent need for the development of novel therapeutic strategy with improvement of clinical efficacy by overcoming resistance as well as concomitant reductions of side effects.
The first goal of this preclinical study was to develop rational combination therapy for the treatment of melanoma by combining metformin and binimetinib. Metformin as antidiabetic drug has been known to be a well-tolerated drug with safety profile and little drug resistance (Scherbakov et al., 2016). It inhibits cell proliferation through activation of AMP-activated protein kinase (AMPK), which could suppress MEK inhibition-induced compensatory activation of PI3K/mTOR signaling pathway (Woodard & Platanias, 2010). Thus, many research groups anticipated that the combination of metformin with MEK inhibitor would give synergistic antitumor activity through simultaneous inhibition of MAPK and PI3K/mTOR pathways. However, the results showed divergent effects of the combination therapy on melanoma cell viability (Vujic et al., 2015). In our previous studies, we suggested that the combination of metformin with trametinib shows the differential growth inhibitory effect; synergistic effects in cell line in which metformin suppresses ERK activity but antagonistic effect in cell line with metformin-induced ERK activation (Lee & Park, 2021). Indeed, the effect of metformin on ERK activity has shown conflicting results in various types of cancer cells (Martin et al., 2012; Morgillo et al., 2013; Della Corte et al., 2016). The present study showed that metformin suppresses ERK activation dose dependently and the combined treatment of metformin with binimetinib leads to synergistic growth inhibition of G361 melanoma cells. These results confirm further our suggestion that ERK activity in response to metformin could serve as a predictive biomarker for development of effective therapeutic regimens when using metformin in combination with other anticancer agents.
While MEK inhibition leads to inhibition of cell proliferation through suppression of MAPK signaling pathway, recent studies reported that it could induce metastatic properties such as migration and invasion (Vultur et al., 2014; Zhao et al., 2017). Our previous study also showed that MEK inhibition with trametinib increases the expression of core EMT regulators such as fibronectin, twist1 and slug in A375 melanoma cells and induces cell invasion and migration (Lee & Park, 2021). In the present study, we tried to confirm this issue again by employing binimetinib, another MEK inhibitor, in G361 melanoma cells. Our present data showed that binimetinib also increases the levels of EMT regulators, and these molecular changes functionally well correlated with the increase of melanoma cell migration. On the contrary, metformin remarkably reduced EMT regulators, which is in agreement with previous reports by other research groups (Cerezo et al., 2013; Tseng et al., 2019). Undoubtedly, the increased metastatic behavior in response to MEK inhibition could be therapeutically a serious obstacle to the effective treatment of melanoma. Thus, we extended the findings to the combination of metformin with binimetinib, and found that metformin counteracts the pro-migratory activity of binimetinib. These findings support the premise that metformin might be useful as a potential anticancer agent for melanoma patients via not only suppression of cell proliferation but also inhibition of metastasis.
Our finding that binimetinib leads to enhancement of cell migration is inconsistent with the report of Ryabaya et al. (2019) in which binimetinib rather inhibits melanoma cell migration. The reason for this apparent contradiction between two studies is not clear, but we suppose that the discrepancy might reside in different treatment regimens of binimetinib. They used binimetinib at 2 μM, whereas we applied the increasing concentrations of binimetinib from 5 nM up to 100 nM. Moreover, we found that binimetinib at low doses (5–25 nM) enhances cell migration in dose-dependent manner, then retards the extent of cell migration at 50 nM, and does not affect cell migration at 100 nM. These results suggest that inhibition of cell migration following treatment with 2 μM of binimetinib could result from apoptosis, which they showed by flow cytometry analysis using Annexin V/PI double staining and increased level of cleaved PARP in Western blot. Further studies by using the same melanoma cell line will be needed to elucidate this issue.
In summary, the present preclinical study shows the first in vitro results that combination of metformin with binimetinib might be a promising therapeutic option for treatment of patients with melanoma. In particular, our data support our previous suggestion that differential alteration of ERK activity in response to metformin could serve as a predictive biomarker for the efficacy of metformin monotherapy or combination therapies with other antitumor drugs. Further in vivo studies using mouse xenograft models and clinical trials are required to better elucidate the combined effect of metformin with binimetinib on antitumor activity in melanoma.

