INTRODUCTION
The luteinizing hormone receptor (LHR) is a member of the rhodopsin-like subfamily of G protein-coupled receptors (GPCRs), one of the largest gene families (Kudo et al., 1996), and mediates the internalization of its two naturally occurring agonists, lutropin and choriogonadotropin (CG) (Min et al., 1998). The LHR gene has been associated with an abundance of naturally occurring mutations related to reproductive failure in humans and mice (Tao et al., 2000; Zhang et al., 2005, 2007; Tao, 2006; Meehan & Narayan, 2007; McGee & Narayan, 2013).
The LHR is composed of a serpentine region containing the seven transmembrane helices typical of GPCRs, as well as a large extracellular domain that confers the high affinity binding of hormones (Simoni et al., 1997; Ascoli et al., 2002). Gonadotropin receptors, including follicle-stimulating hormone (FSH) receptors, are highly homologous in the transmembrane region. In the past several years, numerous human luteinizing hormone receptor (hLHR) mutations have been identified in young boys with gonadotropin-independent precocious puberty (Shenker et al., 1993; Themmen & Huhtaniemi, 2000; Zhang et al., 2005, 2007; Latronico & Segaloff, 2007).
Most of these mutants were divided into two groups: activating and inactivating. The activating mutants have been reported as cells expressing the mutant and displayed remarkable basal cyclic AMP (cAMP) levels. Specifically, cells expressing the L457R mutant were unresponsive to hormonal stimulation (Zhang et al., 2005, 2007). Inactivating receptors were found to be signal-impairing mutations (Dhanwada et al., 1996; Min et al., 1998).
GPCR signal transduction including glycoprotein hormone receptors, has been studied in detail with respect to the loss of cell surface receptors, internalization, and recycling (Bhaskaran & Ascoli, 2005; Jacobsen et al., 2017; Foster & Bauner-Osborne, 2018). Recent research studies on eel glycoprotein hormones and receptors from our laboratory have elucidated the signal transduction of the eel LHR (Byambaragchaa et al., 2018a,b) and eel follicle-stimulating hormone receptor (FSHR; Kim et al., 2016, 2018, 2019) on deglycosylated ligands. We also first reported in fish species that the eel FSHR-D540G mutant exhibited a high increase in the basal cAMP response (Byambaragchaa et al., 2020). Although the activation effects of these mutants have been relatively well demonstrated in hLHR, very little is known about signal transduction leading to activation and inactivation in fish LHR. The basal cAMP increase in activating mutants is also not well understood.
Thus, this study aimed to determine these mechanisms by analyzing two constitutively activating (L469R, and D590Y in eel LHR), and two inactivating (D417N and Y558F in eel LHR) mutations, which were highly conserved regions of transmembrane domains among the mammalian LHRs.
MATERIALS AND METHODS
Polymerase chain reaction (PCR) reagents and endonucleases were purchased from Takara (Osaka, Japan). Oligonucleotides were synthesized by Genotech (Daejeon, Korea). The pcDNA3 mammalian expression vector, Chinese hamster ovary (CHO)-suspension (CHO-S) cells, MAX transfection reagent, LipofectamineTM-3000, Freestyle CHO medium, and antibiotics (penicillin and streptomycin) were obtained from Invitrogen (Carlsbad, CA, USA). The pGEM-T easy cloning vector was purchased from Promega (Madison, WI, USA). Monoclonal antibodies (5A11, 11A8, and 14F5) for recombinant eel LH (rec-eel LH) analysis and rec-eel LH from CHO-S cells were produced in our laboratory, as previously reported (Lee et al., 2021). HEK 293 cells were obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea). QIAprep-Spin plasmid kits were purchased from Qiagen (Hilden, Germany). Spinner flasks for cell culture were purchased from Corning (Corning, NY, USA). Centrifugal Filter Devices for the concentration of rec-eel LH hormone were purchased from Amicon Bio (Billerica, MA, USA). All other reagents used in this experiment were obtained from Sigma-Aldrich (St. Louis, MO, USA) or Wako Pure Chemicals (Osaka, Japan). The procedures and protocols used in this study were reviewed and approved in accordance with the guidelines of the Hankyong National University Committee (Number: 2018-03-01).
The wild-type eel LHR cDNA was cloned from eel ovaries and testes, as previously reported (Byambaragchaa et al., 2020) and subcloned into pcDNA3.1. Mutagenesis was performed using the PCR overlap extension method (Lee et al., 2021). Briefly, two different sets of PCR primers were used to amplify each mutant fragment. The first set of fragments was amplified using the forward and reverse primers (mutation primer). The second set of fragments was then amplified using forward (mutation primer) and reverse primers. The amplified fragments were used as templates to amplify the completely mutated fragments. Full-length PCR products were cloned into a pGEM-T easy vector. Plasmids were extracted and sequenced to confirm the presence of the mutations. The cDNAs encoding wild-type and mutant eel LHR were digested with the Eco RI and Xho I restriction enzymes. The resulting fragments were then ligated into the pcDNA3.1 and pCORON1000 SP VSV-G expression vectors, as previously described (Byambaragchaa et al., 2018, 2021a). Finally, we constructed a total of five receptor genes, including WT eel LHR, mutant eel LHR-L469R, LHR-D590Y, LHR-D417N, and LHR-Y558F.
For ligand production, the recombinant eel LH (rec-eel LH) expression vector was transfected into CHO-S cells using the FreeStyle™ MAX reagent transfection method according to the manufacturer’s instructions, as previously reported in our lab (Byambaragchaa et al., 2018a). The MAX Reagent and plasmid were diluted and gently mixed by inverting the tube. After 10 min at 25°C to allow the formation of complexes, the complexes were added to 200 mL of cell-containing medium, indicating that the cell density was approximately 1.2–1.5×106 cells/mL. Culture media were collected on day 7 after transfection and freezing at −80°C. Rec-eel LH was quantified using a double-sandwich enzyme-linked immunosorbent assay (ELISA) performed in plates coated with the 5A11 monoclonal antibody, as previously described (Kim et al., 2016).
The HEK 293 cells were grown to 80%–90% confluence in 6-well plates, and the plasmid DNAs were transfected using Lipofectamine reagent. After the diluted DNA had been combined with Lipofectamine reagent, and gently mixed by inverting the tube. The mixture was incubated for 20 min at 25°C to allow the formation of complexes. The cells were then washed with Opti-MEM, and the DNA-Lipofectamine complex was added to each well. After 5 h, the medium containing 20% fetal bovine serum was added. The HEK 293 cells were used to analyze surface receptor loss.
Loss of eel LHR from the cell surface was assessed using an ELISA as previously described (Byambaragchaa et al., 2020, 2021b; Mundell et al., 2010). We excluded the M410T mutant, which displayed the lowest basal cAMP response among the activating mutants. Accordingly, we characterized two activating (L469R and D590Y ) and two inactivating (D417N and Y558F) mutants for the loss of receptors from the cell surface. Cells were plated at a density of 6×105 cells per 60 mm dish, and then split into 96-well dishes (1×104 cells) coated with poly-D-lysine 24 h post-transfection. For the loss of cell surface receptors, cells were preincubated with 500 ng/mL rec-eel LH for the time-dependent tests (5, 15, 30, and 60 min). Briefly, cells were fixed in 4% paraformaldehyde in Dulbecco’s PBS for 5 min at 25°C. After washing three times with Dulbecco’s PBS, all wells were incubated with a blocking solution (TBS with 1% BSA) for 30 min. The primary antibody reaction was performed using a rabbit anti-VSVG antibody (1/1,000; Abcam, Cambridge, MA, USA), followed by incubation with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1,500; Abcam). After washing four times with blocking solution, 80 µL of PBS and 10 µL of SuperSignal ELISA Femto Maximum substrate (Thermo Fisher Scientific, Waltham, MA, USA) was added to each well for detection. Luminescence was measured using a Cytation 3 plate reader (BioTek, Winooski, VT, USA). The expression level of the wild-type receptor was set to 100%. The loss of wild-type and mutant eel FSHRs from the cell surface was calculated by comparing the levels in the presence of rec-eel LH to the levels in the absence of stimulation with the agonist (taken as 0% loss of cell surface receptors).
The MultAlin interface-multiple sequence alignment software was used for the sequencing results. The GraphPad Prism 6.0, was used for the analysis of cell surface loss and GraFit 5.0 (Erithacus Software Limited, Surrey, UK) was used to analyze the stimulation curves. Curves fitted in triplicate were normalized to the background signal measured for mock-transfected cells. One-way ANOVA and Turkey’s multiple comparison tests were used to compare the results between samples using GraphPad Prism 6.0. Differences were considered significant between groups (p<0.05).
RESULTS
The cell surface expression of eel LHR was determined by an ELISA in transiently transfected CHO-K1 cells (Fig. 1). Receptor expression was the same in the cells expressing the activating mutants and inactivating mutants as in those expressing wild-type eel LHR. The expression level of wild-type eel LHR was considered to be 100%, and the expression levels of L469R and D417N were 97% and 101%, respectively, whereas the expression levels of D590Y and Y558F slightly increased to approximately 110% and 106%, respectively.
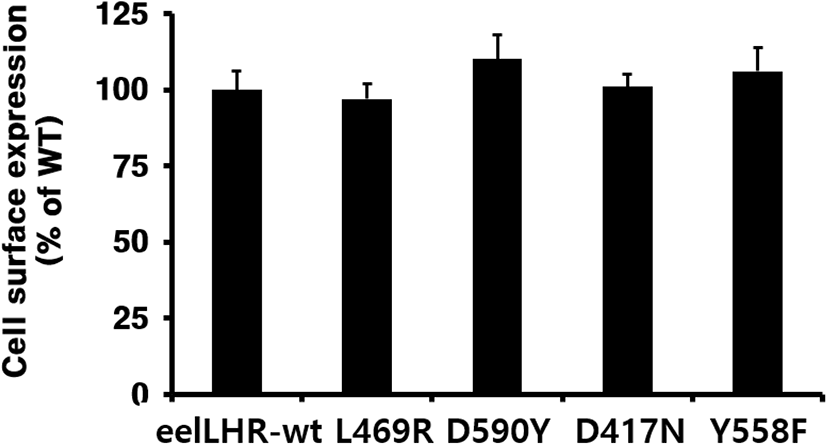
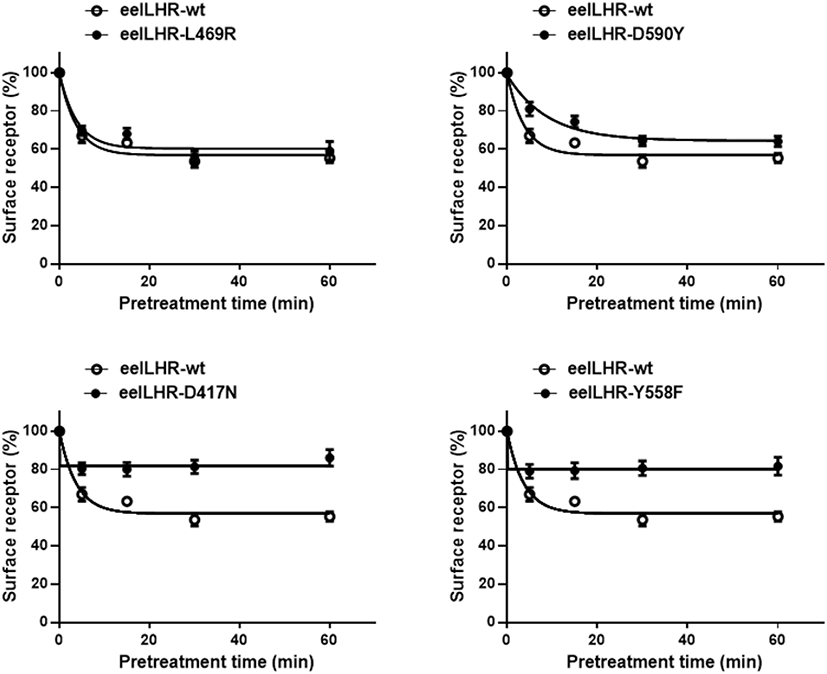
To more accurately quantify the rate of loss of cell surface receptors, we performed experiments in which the loss of receptors from the cell surface was measured in a time-dependent manner in the presence of eel LH. The results of the time-dependent loss of eel LHRs for two activating (L469R and D590Y) and two inactivating (D417N and Y558F) mutations are shown in Fig. 2. The expression of surface receptors in cells harboring the wild-type eel LHR gradually decreased until it reached 55% of the pretreatment value. Similarly, the expression of cell surface receptors in cells expressing the activating mutants (L469R and D590Y) decreased to approximately 70%–80% in the first 5 min and then a slightly decreased in the following 15 min. Finally, the loss of receptors remained between 60% and 64% for 60 min. The surface loss of the receptor was slightly observed in the cells expressing the inactivating mutants (D417N and Y558F). As shown in Fig. 2, the expression of cell surface receptors in cells expressing the D417 and Y558F mutants decreased to approximately 80% in the first 5 min, then remained without a change for 60 min.
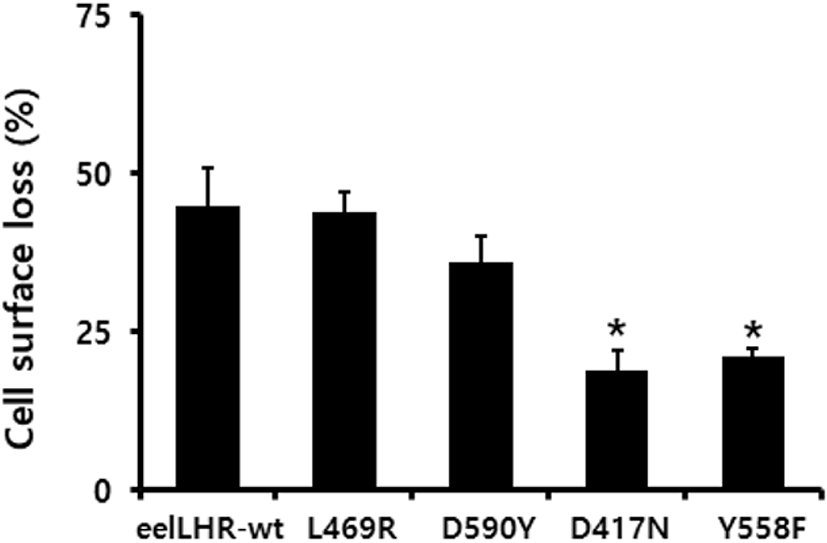
The loss of cell surface receptor expression was measured for 30 min. The results were then expressed as a percentage of the loss of the expression of surface receptors measured in control cells pre-incubated in the absence of the eel LH agonist (considered as 0% loss of surface receptors). Cells expressing wild-type eel LHR treated with eel LH agonist (500 ng/mL) for 30 min exhibited an increased loss (>45 %) of cell surface receptors. In cells harboring the L469R activating mutation, the loss of cell surface receptors was considerably similar (44%) to that observed in the wild type. We also found that cells expressing the D590Y mutant exhibited slower expression rates (36%) than those expressing the corresponding wild-type receptor. In cells expressing the inactivating D417N and Y557F mutants, the loss of cell surface receptors induced upon stimulation with eel LH agonist was demonstrated to be only 19% and 21%, respectively (Fig. 3).
The rate of formation of the agonist-receptor complexes induced by the constitutively activating and inactivating mutants of eel LHR are presented in Table 1. The rates of loss of cell surface agonist-receptor complexes in both the wild-type and activating mutant receptors were observed to be very rapid (2.6–6.2 min), as shown in Table 1. However, the D417N and Y558F inactivating mutants showed almost no loss of cell surface receptors. These data clearly show that the two inactivating mutations (i.e., eel LHR-D417N and -Y558F) significantly reduced the rate of cell surface loss of eel LHR, whereas activating mutations (i.e., eel LHR-L469R and -D590Y) enhanced the rate of cell surface loss of LHR.
Data were fitted to one phase exponential decay curves to obtain values of t1/2 and plateau (i.e. maximum reduction). Data from individual experiments performed in triplicate out of 3 independent experiments.
LHR, luteinizing hormone receptor
DISCUSSION
The present study was designed to determine the possibility that the activation/inactivation of eel LHRs might be necessary for the loss of cell surface receptors induced by ligand agonists.
Several previous studies have reported activating/inactivating mutants, including hLHR and rat LHR (rLHR) (Yano et al., 1996; Latronico et al., 1998; Galet & Ascoli, 2006; Latronico & Segaloff, 2007; Zhang et al., 2007). Recently, we also reported that the activating mutants in eel FSHR and equine LH/CGR displayed an increased basal cAMP response without agonist, but inactivating mutants were completely impaired in signal transduction (Byambaragchaa et al., 2020, 2021b). Previous results from our colleagues have also shown that basal cAMP responses in cells expressing rLHR-L435R (equivalent to L469R in eel LHR) displayed a 47-fold increase in the absence of agonist, without leading to a further increase in the cAMP response following stimulation by hCG (Min et al., 1998). Previous studies also have reported that hLHR-L457R and hFSHR-L460R mutants resulted in strong constitutive activation (Tao et al., 2000; Zhang et al., 2007).
Our data (Byambaragchaa et al., 2020, 2021b,c) are consistent with previous results, indicating that the L469R mutant displayed a high basal cAMP response (Latronico et al., 1998; Min et al., 1998; Zhang et al., 2005, 2007; Latronico & Segaloff, 2007). However, the mechanisms underlying the increase in basal cAMP production remains unclear. Thus, we analyzed the correlation between basal cAMP response and cell surface loss of receptors in the constitutively activating mutants (eelLHR-L469R and -D590Y) and inactivating mutants (eelLHR-D417N and -Y558F).
Based on the activation model, we expected the rate of loss of the L469R and D590Y mutated cell surface receptors to be the same as the rate of loss of receptors in cells expressing the wild-type receptor. The results presented here revealed that the rate of loss of the constitutively active mutants (L469R and D590Y ) from the cell surface following a 5 min preincubation with agonist was decreased by approximately 30%, whereas the loss of the D417N and Y558F inactivating mutants was dramatically slower than that of the wild-type receptor. These results were also in agreement with the findings regarding the cAMP response following treatment with the agonist, which was shown to markedly increase the activation hLHR mutants (Bhaskaran & Ascoli, 2005; Jacobsen et al., 2017) and hFSHR (Tao et al., 2000), whereas it was impaired in inactivating eel FSHR mutants (Byambaragchaa et al., 2020) and equine FSHR (Byambaragchaa et al., 2021d). Our results are consistent with previous data reported by our colleagues studying equivalent mutations in rLHR, in which they demonstrated that two signaling-impairing mutations, rLHR-D383N and rLHR-Y524F, decreased the rate of internalization of hCG (Dhanwada et al., 1996), whereas hLHR-L460R, rLHR-L435R and rLHR-D556Y activating mutants enhanced the internalization rate (Min et al., 1998; Bhaskaran & Asoli, 2005; Latronico & Segaloff, 2007). The results presented here showed for the first time that two constitutively activating mutants of eel LHR were able to conclusively affect the loss of these receptors from the cell surface. In the hLHR L457R, the naturally occurring mutant L457R causes constitutive activation as a result of the interaction of the introduced arginine residue in helix 3 with Asp-578 (Zhang et al., 2005). Of the many constitutively active mutants of the hLHR that have been reported, the L457R mutant is of particular interest for several reasons, demonstrating that the L457R mutant has a particularly strong constitutive activity (Zhang et al., 2005) followed by eel FSHR (Byambaragchaa et al., 2020), rLHR (Min et al., 1998), and eel LHR (Byambaragchaa et al., 2021c). However, the hL457R mutant (L469R in eel LHR) is not as active as the agonist-occupied wild type LHR, suggesting that L457R likely stabilizes an intermediate state of activation of the receptor, indicating that the mutant is unresponsive to further hormonal stimulation despite its ability to bind hormones with high affinity (Latronico & Segaloff, 2007). In the present study, the eel LHR-L469R mutant exhibited faster cell surface loss of receptors than wild-type eel LHR. Thus, we suggest that basal cAMP production causes a rapid loss of receptors from the cell membrane. However, the maximal cAMP responsiveness in the L457R mutant was only half that of the wild-type receptor. Therefore, the occurrence of differences in the basal cAMP level and maximal cAMP levels are difficult to understand in this mutant.
In conclusion, this study showed that the rate of loss of the D590Y mutant from the cell surface was slightly slower, whereas that of the L469R mutant was similar to that of the wild-type receptor. The loss of the D417N and Y558F inactivating mutants from the cell surface was considerably slower than that of the agonist-occupied wild-type receptor. Our study further highlighted the importance of the crucial transmembrane regions of eel LHR in agonist-induced loss of eel LHRs from the cell surface.

