INTRODUCTION
Kisspeptins (formerly known as metastin) are proteins encoded by the KISS1 gene in mammals including human (Dungan et al., 2006). Kisspeptins are ligands of the G-protein coupled receptor, GPR54(also denoted by kiss1r), and the kisspeptin-GPR54 signaling has an important role in initiating secretion of hypothalamic gonadotropin-releasing hormone (GnRH) at puberty (Dungan et al., 2007). GnRH neuron–specific Kiss1r knockout (GKirKO) mice were infertile, showing low serum LH and FSH levels (Novaira et al., 2014). Furthermore, male human plasma kisspeptin levels were significantly co-related with hypogonadism (Kotani et al., 2014) and positively associated with semen quality (Zou et al., 2019).
Besides the hypothalamus, interestingly, ample of evidence indicates that the kisspeptin-GPR54 system works in gonads. The extrahypothalamic expressions of kisspeptin in ovary have been found in human (Gaytan et al., 2009; Garcia-Ortega et al., 2014) and rodents such as rat (Castellano et al., 2006; Zhou et al., 2014) and mice (Hsu et al., 2014; Merhi et al., 2016). It is also evident that the presence of the testicular kisspeptin-GPR54 in human (Pinto et al., 2012), monkey (Irfan et al., 2016), rat (Zhou et al., 2014) and mice (Funes et al., 2003; Mei et al., 2013; Salehi et al., 2015).
Hamsters, another potent experimental rodent, also have a kisspeptin-GPR54 system in their ovaries. So far, however, the presence of testicular kisspeptin-GPR54 system remains to be solved. The present study was undertaken to determine whether the kisspeptin is expressed in hamster testis. To do this, the presence of transcripts and proteins for kisspeptin was tested by using reverse transcription-polymerase chain reactions (RT-PCRs) and immunohistochemistry (IHC), respectively.
MATERIALS AND METHODS
Mature male Syrian hamsters (Mesocricetus auratus) were used in this investigation. They were housed in animal facility with the lighting scheme that was regulated by the plug-in timer. The timer was set by lights of 14 h and darkness of 10 h where the reproductive activity of the hamsters is actively maintained (light turned on 6 AM). The animals were fed with standard laboratory mouse chow and tap water ad libitum. The animal protocols were approved by the Animal Care and Use Committees at Sangmyung University (approval number R-2101-2). All the animals received humane care in accordance with the guides for animal experiments of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
The mature hamsters were sacrificed by decapitation. The skin covering the head was peeled off to expose upside of the skull. The temporal bones were cut in both sides by the anatomical scissors, positioning one side of the anatomical scissors pricked through the spinal cord and the other side exterior the occipital bones. The skull was eliminated, and the brain was leaned back by cutting out the optic nerves. The hypothalamus in the shape of oval and convex region at the center of the whole brain was lifted out by the concave scissors. The testicles were cut out following excision of the skin of scrotal sac. Animals were sacrificed, and sample collections were done between 18:00-19:00 pm. The tissues were immediately soaked in the RNA isolation solution, stored at −70°C until RT-PCRs. Some tissues were fixed in paraformaldehyde (4%) for histological study.
Total RNAs were isolated from samples using the acid guanidinium thiocyanate-phenol-chloroform (AGPC) procedure (Chomczynski & Sacchi, 1987). To five volumes of the RNA extraction solution containing the sample were added 1 volume of 3 M sodium acetate (pH 5.2, Bioneer, Daejeon, Korea) and 6 volumes of PCI (pH 4.3, 5:1 Phenol:Chloroform, Bioneer) in a 1.5 mL tube (Hankuk Bioscience, Seongnam, Korea). Mixed samples by inverting were centrifuged at 14,000×g for 25 min in cooling centrifuge (Hanil, Gimpo, Korea) at 4°C. The supernatant was carefully transferred to a new tube and the same volume of isopropanol (Merck, Darmstadt, Germany) was added. The samples were mixed and placed over an hour at −20°C. The samples were centrifuged prior to the supernatant removed. The precipitated pellet was added to the RNA extraction solution and this process was repeated once more. Then the RNA pellet was washed with 1 mL of 70% ethanol (Merck) twice by centrifugation. The final pellet was resuspended in diethylpyrocarbonate (DEPC, Sigma-Aldrich, St. Louis, MO, USA) -treated distilled water. The samples were stored at −70°C before using.
Total RNAs were reverse transcribed using Maxime™ RT PreMix (Oligo-dT, iNtRON, Seongnam, Korea). The PCR of cDNAs were performed by Taq PCR Master Mix (Beams, Tokyo, Japan) according to each condition. Primer pairs (Table 1) were designed using basic local alignment search tool (BLAST) and an order was placed for the oligos with Bioneer. The reactions were subjected to MultiGene™ OptiMax Thermal Cycler (Labnet, Edison, NJ, USA). The PCR products were confirmed by 1.5% or 3% agarose gel with ethidium bromide (EtBr, Sigma-Aldrich) into an electrophoresis system (OPTIMA, Green Bay, WI, USA) and the cDNA of the putative kisspeptin PCR product was sequenced (MACROGEN, Seoul, Korea).
Fixed testes were dehydrated in graded concentrations of ethanol (70%, 80%, 90%, 95%, and 100%; Duksan, Ansan, Korea) for 1 h 30 min in each with gentle shaking and soaked in absolute ethanol overnight. The tissues were immersed in xylene (Samchun Chemical, Seoul, Korea) for 30 min, 3 times and in paraffin at 56°C for 30 min, 3 times. The tissues were embedded in paraffin and sectioned (Microm, Walldorf, Germany) at 5 μm. The samples were attached on microscope slides and the slides were stained with hematoxylin (Sigma-Aldrich) for 5 min and eosin (Across, Carson, CA, USA) for 5 min, respectively.
The slides were dewaxed in xylene for 5 min, 3 times and hydrated in 100%, 95%, 90%, 80%, and 70% ethanol for 5 min each. The specimens were washed in PBS for 5 min, 4 times and immersed in hydrogen peroxide solution [10% H2O2 (Daejung, Siheung, Korea) and 10% Methanol (Merck)] in PBS for 20 min, and washed in PBS 2 times. The slides were incubated with blocking serum [2% BSA (Bio PURE, Cambridge, MA, USA) and 2% normal goat serum (Vector, Olean, NY, USA)] in PBS for 1 h and incubated with diluted primary antibody (1:50, for Kiss1 Polyclonal antibody, MyBioSource, San Diego, CA, USA) in blocking serum overnight. And then, the slides were washed 2 times and incubated with biotinylated goat anti-rabbit IgG secondary antibody (Vector) for 1 h (in the case of Cgα, overnight) and washed in PBS 2 times. The samples were incubated with avidin-biotin-peroxidase complex using the ABC kit (Vector) for 40 min and washed 2 times. The chromogenic reaction was progressed using the DAB kit (Vector) and stopped by washing in tap water. The slides were stained in hematoxylin (Vector) for 7 min and dehydrated in graded ethanol (70%, 80%, 90%, 95%, and 100%) for 3 min each and then cleaned in xylene for 5 min, 3 times. The slides were mounted with Permount (Fisher Scientific, Waltham, MA, USA). The images were captured with the BX51 Microscope & DP70 Digital Camera System (Olympus, Tokyo, Japan).
RESULTS
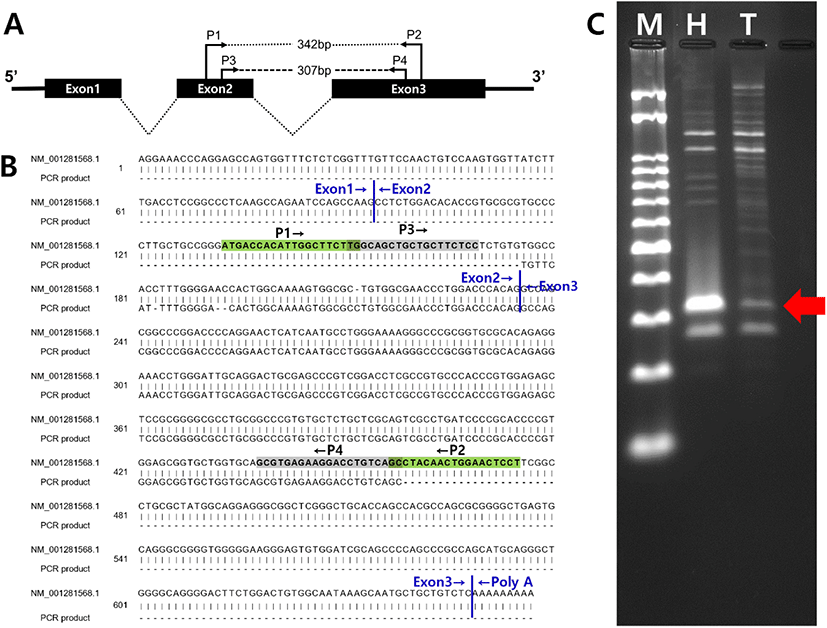
Total RNAs from hamster hypothalamus (control) and testis (experimental group) were reverse transcribed with olio-dT, and the cDNAs were applied to amplification of hamster kisspeptin cDNA by PCR. First PCR using primer set P1 and P2 failed to amplify cDNA of the correct size (data not shown). The 1st PCR products were diluted and applied to 2nd round (nest) PCR using primer set P3 and P4. After the nest PCR, two cDNA products (320 and 280 bp, respectively) were detected by 3% agarose gel electrophoresis (Fig. 1A). Following sequencing revealed that the 320 bp product was correctly amplified from hamster kisspeptin cDNA (Fig. 1B and C).
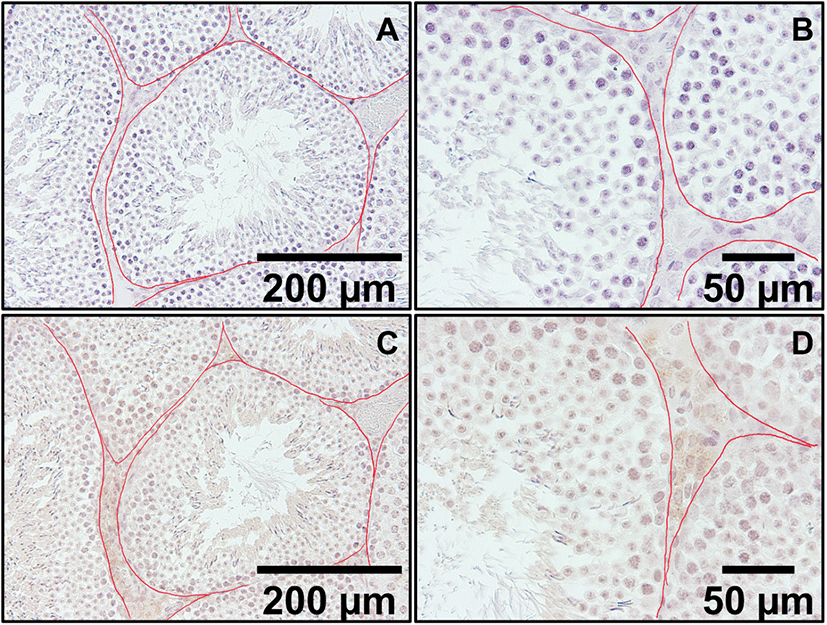
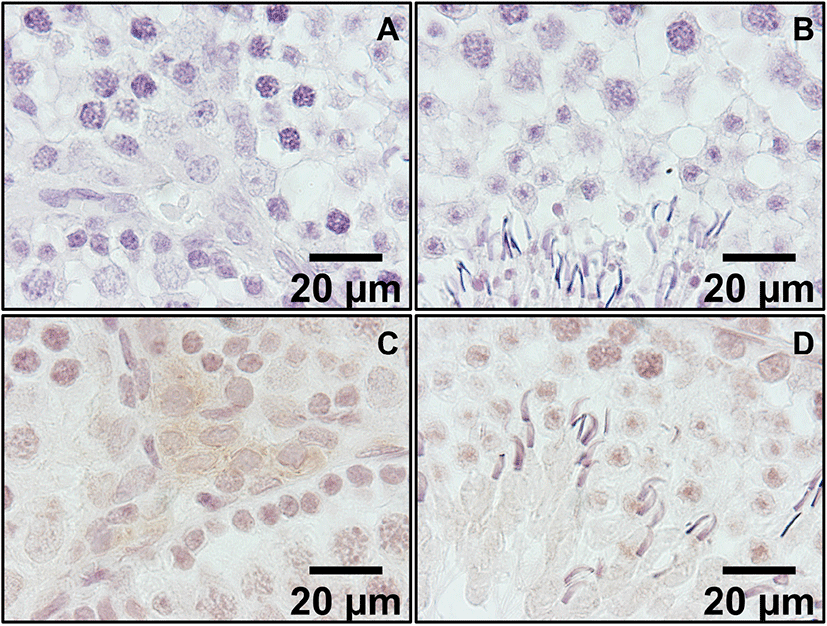
In the low magnification pictures (×200 and ×400), positive staining was present in Leydig cells located outside the seminiferous tubule (Fig. 2C and D). In control sections, same conditions without secondary antibodies resulted in no positive signals. In high magnification picture (×1,000), positive signals were found in the cytoplasm of Leydig cells and some light signals were observed in round spermatids and elongated spermatids (Fig. 3C and D, respectively). Kisspeptin immunostaining was undetectable or barely detectable in majority of cells in the seminiferous tubules. Interestingly, but it was detected partially in the growing acrosomes in elongated spermatids, which are the transitional cells during spermiogenesis (Fig. 3D).
DISCUSSION
Since the pioneering work of Harris on the prediction of hypothalamic releasing hormone which are conveyed from the median eminence to the pituitary gland through the hypophyseal portal vessel to control the synthesis and secretion of pituitary hormones such as gonadotropins (Harris, 1948; Uenoyama et al., 2016), the hypothalamic-pituitary-gonad (HPG) hormone axis has been a central concept in understanding mammalian reproductive phenomena; specifically, it is a feedback control system by hypothalamic GnRH, pituitary gonadotropins (FSH and LH), and gonadal sex steroids (Plant, 2015). Currently, the hormone axis concept has developed very complex; for example, the hypothalamus level was expanded by the kisspeptin-GPR54-GnRH system (Uenoyama et al., 2016).
In rodent hypothalamus, 2 distinct populations of Kiss1 neurons, namely arcuate nucleus (Kiss1ARH) neurons and anteroventral periventricular and periventricular nucleus (Kiss1AVPV/PeN) neurons in rodents, both of which excite GnRH neurons via kisspeptin release but are differentially regulated by ovarian steroids, particularly estrogen (Rønnekleiv et al., 2022). Indeed, this estrogen-induced kisspeptin signal is now widely accepted as a key regulator of positive feedback which is responsible for the LH surge in female HPO axis (Ozawa, 2021). Two transgenic mice studies provided strong evidence. Firstly, when GPR54- and Kiss1-null mice were examined, all wild-type littermates exhibited LH surges in approximately 50% of their GnRH neurons, none of the mutant mice from either line showed an LH surge or any GnRH neurons with c-FOS (Clarkson et al., 2008). Secondly, wild-type female mice consistently display LH surges under multiple hormonal paradigms, whereas Kiss1r-null mice do not, indicating that kisspeptin-Kiss1r signaling is mandatory for GnRH/LH surge induction (Dror et al., 2013).
Furthermore, kisspeptin-GPR54 system is found at other HPG axis levels, in ovary and testis. Despite this fundamental role in the central nervous system, kisspeptin is expressed in different tissues, including the ovary (Castellano et al., 2006; Bhattacharya & Babwah, 2015; Fernandois et al., 2017) and testis (Pinilla et al., 2012; Chiang et al., 2020). Kisspeptin-GPR54 is involved in rat ovarian follicular development (Fernandois et al., 2016), human oocyte maturation (Jayasena et al., 2014), ewe ovulation (Caraty et al., 2007), rat steroidogenesis (Peng et al., 2013), and in some pathological events such are premature ovarian failure (Gaytan et al., 2014), polycystic ovary syndrome (Tang et al., 2019) and endometriosis (Makri et al., 2012). Fewer studies have been done on the testicular kisspeptin-GPR54 compared to the ovarian kisspeptin-GPR54. The testicular Kisspeptin-GPR54 is involved in formation of mouse spermatozoa (Mei et al., 2013), human sperm hyperactivation (Pinto et al., 2012), rat testicular steroidogenesis (Ayturk et al., 2017), Sertoli cells androgen-binding protein (ABP) production in primate (Irfan et al., 2016).
Syrian hamsters belong to the rodent family, as do other popular lab animals like rats and mice. In many respects rats/mice and hamsters will share functions, so testicular kisspeptin signals in hamster could be fine-tunings of spermatogenetic procedures (Mei et al., 2013) and control of steroidogenesis (Ayturk et al., 2017). On the other hand, the hamster has been the most intensively studied of photoperiodic species, and frequently has been presented as a model for the role of the pineal gland in regulating reproductive activity (Pévet, 1988; Choi & Han, 2010; Choi & Lee, 2012). This species is a long-day breeder and exhibits gonadal regression following at least 8 weeks of exposure to short days—i.e., daylengths of less than 12.5-h illumination/24-h cycle (Elliott, 1976). Indeed, gonadal regression, a phenomenon unique to many vertebrates showing seasonal reproduction, would be a particularly interesting topic not only in basic science but livestock industry. In this respect, Syrian hamsters have a great advantage; well-established animal model, small body-size, presence of artificially induced gonad regression model, etc.
In the present study, we demonstrated that the kisspeptin expression in the testis of Syrian hamster. Further studies on the local role(s) of testicular kisspeptin are expected for a better understanding the physiology of hamster testis, including photoperiodic gonadal regression specifically occurred in hamster gonads.

