INTRODUCTION
The adductor muscle of the Pacific oyster (C. gigas) plays an important role in valve opening and closing in response to stimuli (Poulet et al., 2003). The structure, function, and relaxation and contraction mechanism of the molluscan adductor muscle have been investigated previously by several researchers (Millman, 1967; Morrison, 1996; Poulet et al., 2003). The tension of molluscan muscle is indicative of health, including in oyster (Poulet et al., 2003). In addition, the mollusk adductor muscle is a seasonal energy repository, the status of which differs according to reproductive and environmental factors (Racotta et al., 1998). Energy storage in the adductor muscle is associated with energy demand for gametogenesis (Barber & Blake, 1981). Accumulated energy in the adductor muscle of well-fed bivalves is transferred to the gonads during gametogenesis (Gabbott, 1975), and can be used as an indicator of physiological status. The shell and condition index (CI), gametogenic cycle, and nutrient composition have been used to evaluate the growth and reproduction of bivalves (Mondol et al., 2016; Dridi et al., 2017). Improved growth and maturation of bivalves can enable farmers to increase their profit margins; therefore, it is important to identify the precise mechanisms. It has been known that animal growth, especially in vertebrates is under the regulation of the endocrine system. One of which is the insulin-like growth factor (IGF) system. IGF system is composed of three components, the ligand, receptor and IGF-binding proteins (Wood et al., 2005). In invertebrates, presence of IGF ligands and binding proteins have been described, although clear evidence has not been revealed. Similarly, a large number of insulin-like peptides have been reported, and receptors and signaling systems have been identified accordingly, suggesting the possibility of the existence of an IGF system (Gricourt et al., 2003; Hamano et al., 2005; Jouaux et al., 2012). Previously, Choi et al. (2018) reported on the influence of some members of IGF system-molluscan insulin-related peptide (MIP), C. gigas insulin receptor-related receptor (CIR) and IGF binding protein complex acid labile subunit (IGFBP-ALS)-when estimating growth variation in oysters. Although these genes are involved in muscle growth, their exact relationship with regards to the expression of muscle protein are not yet fully described (Heath et al., 2008). The molluscan muscle contains myosin heavy chain (MHC), filamin-C fragment (FIL-C) and actin (ACT) which are used by the organisms for various functions, such as contraction and locomotion, and is used as an indicative of muscle growth (Millman, 1967; Hevrøy et al., 2006; Zhou et al., 2010).
We hypothesized that the CI affects several major genes of the adductor muscle of C. gigas cultivated with non-hardening spat and that any changes in gene levels affect reproduction and growth. Therefore, we checked the presence of muscle proteins and investigated gene expression of the IGF system in the adductor muscle of diploid and triploid oysters C. gigas during a 1-year period, and the relationship between CI values and expression levels of IGF-related genes in the adductor muscle.
MATERIALS AND METHODS
Forty diploid and twenty triploid C. gigas were obtained monthly from November 2016 to December 2017 from Tongyeong, Gyeongsangnam-do, Korea (34°51'32.34''N, 128°12'23.44''E in a diploid oyster farm, and 34°52'39.66''N, 128°14'38.13''E in a triploid oyster farm). CI and tissue weight rate (TWR) were evaluated using shell length (SL), shell height (SH), shell width (SW), total wet weight (TW), and soft tissue weight (STW). These parameters were measured using Vernier calipers (Mitutoyo, Kawasaki, Japan) and an electrical balance (AJ Vibra, Shinko Denshi, Tokyo, Japan). CI was calculated according to the modified method of Choi & Chang (2003) as CI=STW (g)/SL (mm)×SH (mm)×SW (mm)×1,000. In each group, 5 to 10 oysters were used for protein sequencing; the others were used for reverse transcription polymerase chain reaction (RT-PCR). A piece of adductor muscle was dissected, cut into a 1×1-cm square, frozen in liquid nitrogen, and stored at −75°C until use.
Proteins extracted from diploid oysters were resolved by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and identified by tandem mass spectrometry (MS/MS) and electrospray ionization quadrupole time-of-flight MS/MS (ESI-Q-TOF MS/MS, ABI), as described previously (Choi et al., 2015). Proteins were identified using the NCBI (https://www. ncbi.nlm.nih.gov) and UniProt Knowledgebase (http://www.uniprot.org/uniprot) databases and MASCOT (Matrix Science, London, UK) and FASTA software.
Total RNA was prepared from the adductor muscles of diploid and triploid C. gigas using Trans Zol-UP (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. cDNA was synthesized using a first-strand cDNA synthesis kit (PrimeScript™, TaKaRa, Otsu, Japan) following the manufacturer’s protocol. RT-PCR was performed using Emerald Amp® GT PCR Master Mix (TaKaRa-Bio, Otsu, Japan) with C. gigas cDNA as the template. The primer sequences are shown in Table 1. Elongation factor-1α (EF-1α) was amplified using 25 cycles of 30 s at 94°C for denaturation, 30 s at 55°C for annealing, and 30 s at 72°C for elongation. The other genes were amplified using 25 cycles of 30 s at 94°C for denaturation, 1 min at 60°C for annealing, and 1 min at 72°C for elongation. The PCR products were analyzed by electrophoresis in 1% agarose gels and densitometry using GeneTools ver. 4.03 (Syngene, Cambridge, UK).
RT-PCR, reverse transcription polymerase chain reaction; F, forward; R, reverse; ACT2, actin 2; EF-1α, elongation factor-1α; FIL-C, filamin-C fragment; MHC, myosin heavy chain; CIR, Crassostrea gigas insulin receptor-related receptor; IGFBP-ALS, insulin-like growth factor binding protein complex acid labile subunit; MIP, molluscan insulin-related peptide.
Growth data are expressed as means±SD and RT-PCR data are given as mean±SEM. All data were evaluated by one-way analysis of variance (Statistical Package for the Social Sciences, ver. 10.0; SPSS, Chicago, IL, USA). Significant differences between means were identified using Duncan’s multiple-range test (p<0.05).
RESULTS
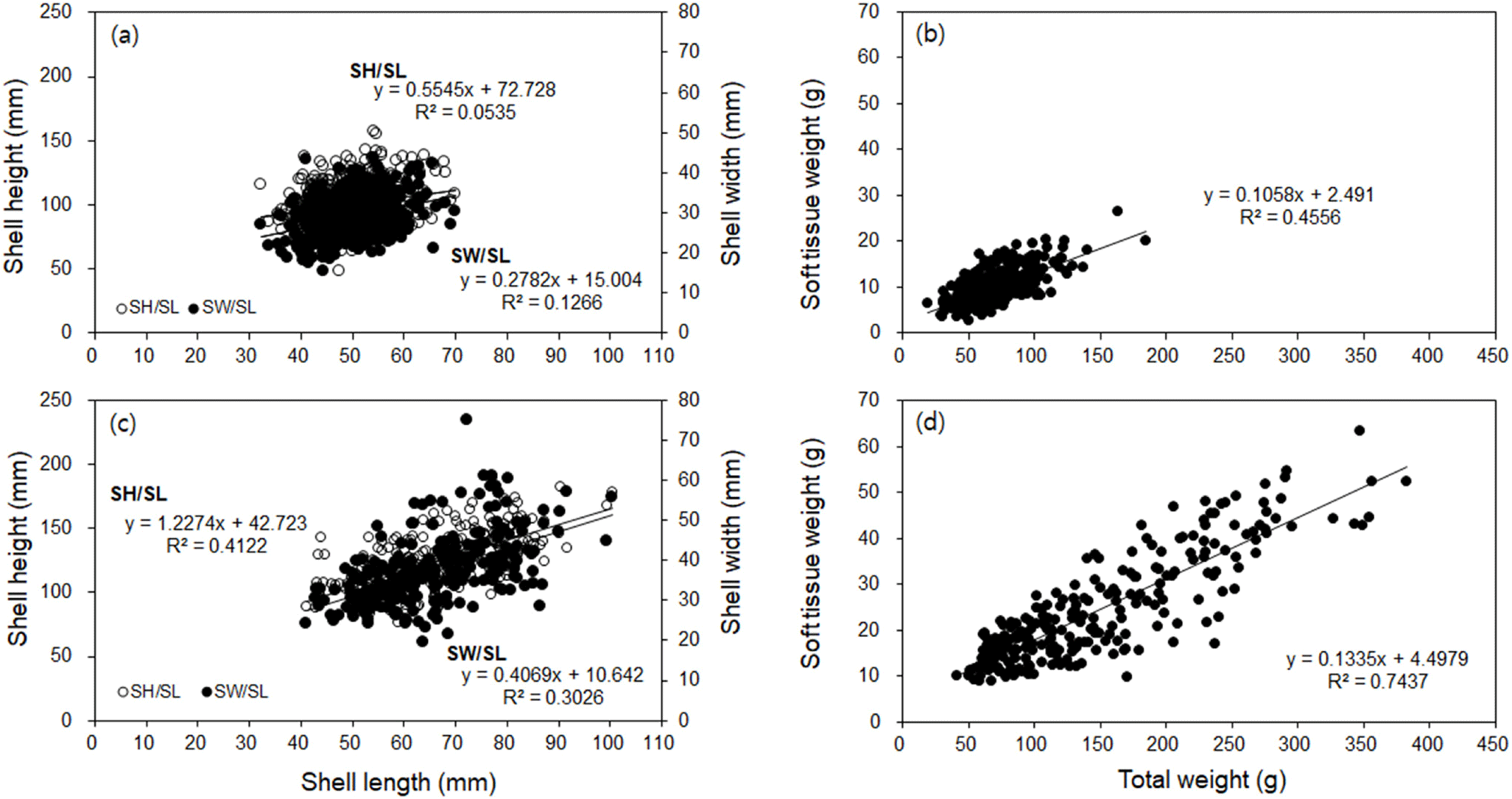
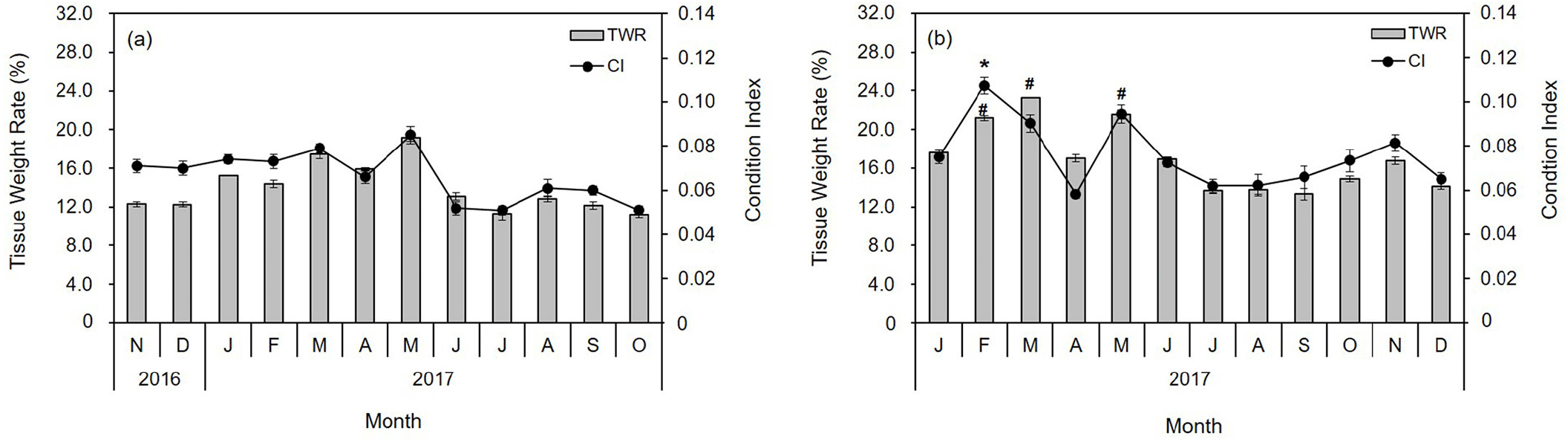
The water temperature and salinity level at the diploid and triploid oyster farms on the coast of Tongyeong were 10.7°C–27.7°C and 32.5–34.0 psu, and 8.9°C–26.9°C and 32.0–33.9 psu, respectively. The ratio of SH to SL ranged from 1.02 to 3.58 (mean 2.05±0.37) and from 1.28 to 3.25 (mean 1.90±0.30) in diploid and triploid oysters, respectively (Fig. 1A and 1C). The ratio of SW to SL ranged from 0.36 to 0.86 (mean 0.58±0.10), and from 0.30 to 1.04 (mean 0.57±0.11) in diploid and triploid oysters, respectively (Fig. 1A and 1C). The ratio of STW to TW ranged from 0.06 to 0.34 (mean 0.14±0.05) and 0.06 to 0.31 (mean 0.17±0.05) in diploid and triploid oysters, respectively (Fig. 1B and 1D). Thus, the growth rates of STW/TW, SH/SL, and SW/SL were positive in both oyster groups, while the size and weight were greater in triploid oysters than in diploid oysters (Fig. 1). In addition, the monthly changes in CI and TWR values were similar (Fig. 2). The highest CI and TWR values were 0.085±0.01 and 19.96±3.16, respectively, in May (p<0.01) and decreased sharply in June and July, in diploid oysters. In comparison, in triploids, the CI was significantly high at 0.107±0.00 in February (p<0.05), and the TWR was significantly high in February, March, and May (p<0.05).
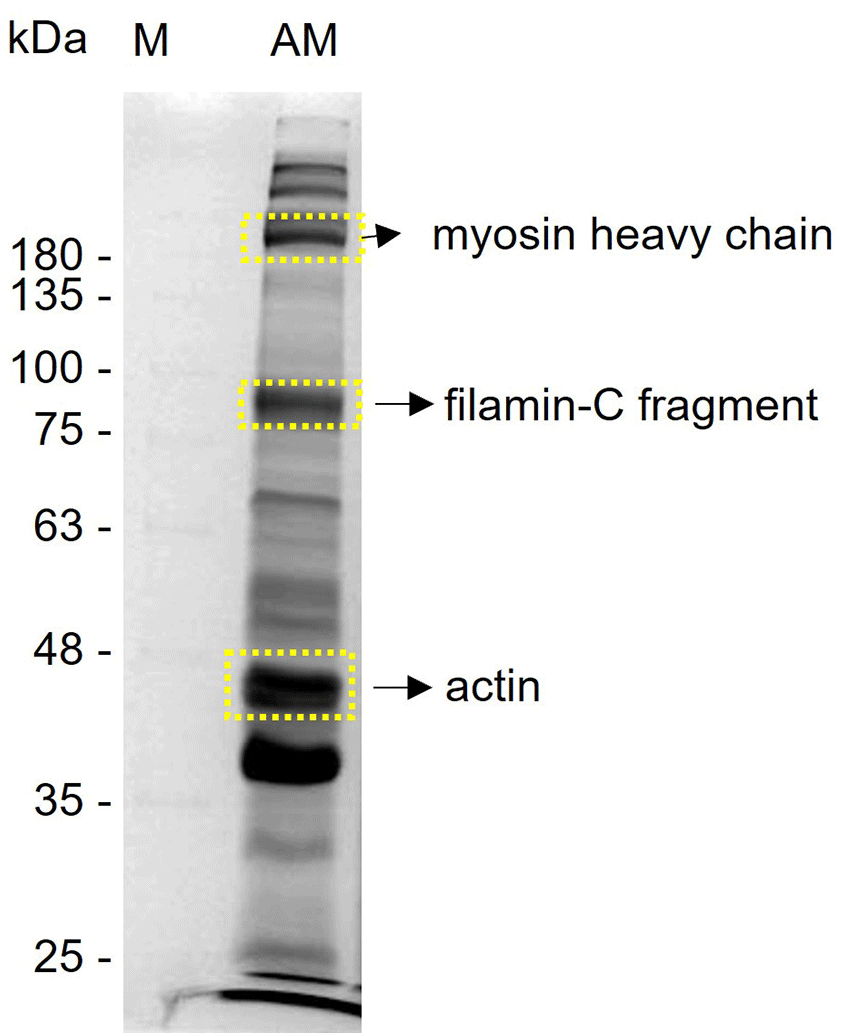
Three proteins were identified as 229 kDa MHC striated muscle, 90 kDa FIL-C, and 42 kDa actin 2 (ACT2) (Table 2, Fig. 3) in the adductor muscle by comparing the predicted amino acid sequences against the NCBI and UniProt protein databases.
MS, mass spectrometry.
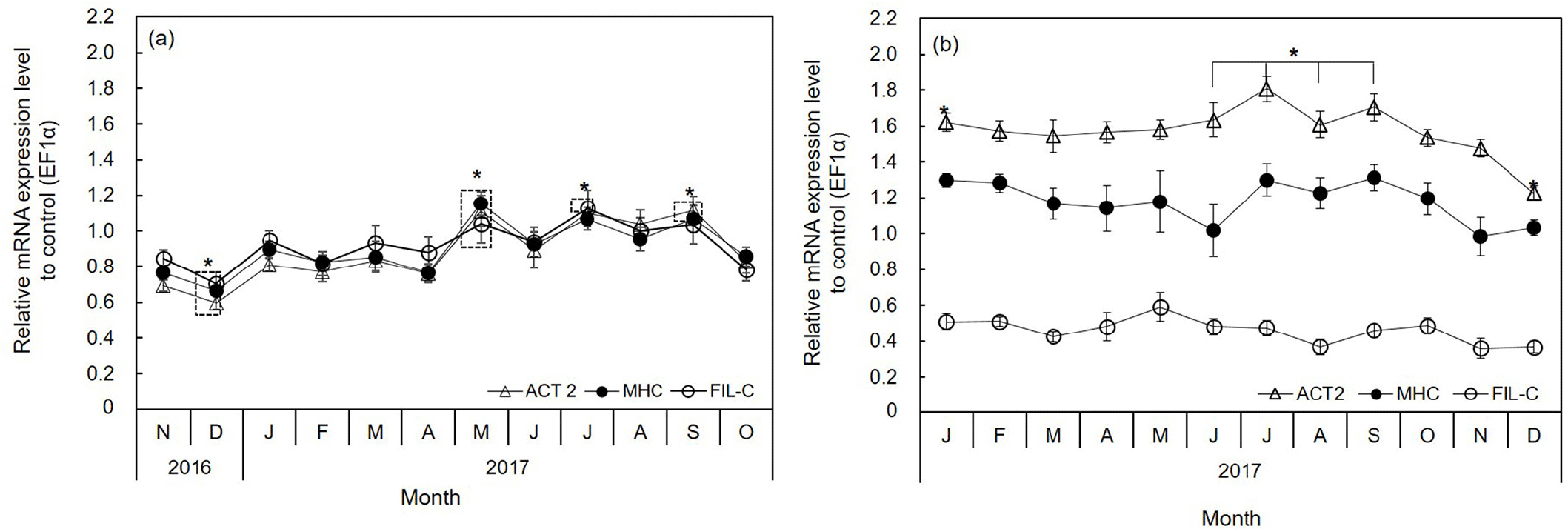
MHC, FIL-C, and ACT2 were expressed in the adductor muscle; however, their expression levels varied monthly. The expression levels of these genes in diploid oysters decreased significantly in December (p<0.01) and increased significantly in May (p<0.01) (Fig. 4A). Moreover, their expression sharply increased in December to January and April to May. In January and May, the expression levels increased 1.3- to 1.5-fold for MHC, 1.2- to 1.4-fold for FIL-C, and 1.4- to 1.5- fold for ACT2 compared to the preceding month (Fig. 4A). The obvious difference between diploid and triploid oysters was that the expression of MHC, FIL-C, and ACT2 was similar in the diploid, while it depended on mRNA levels in the triploid and followed the order ACT2>MHC>FIL-C (Fig. 4).
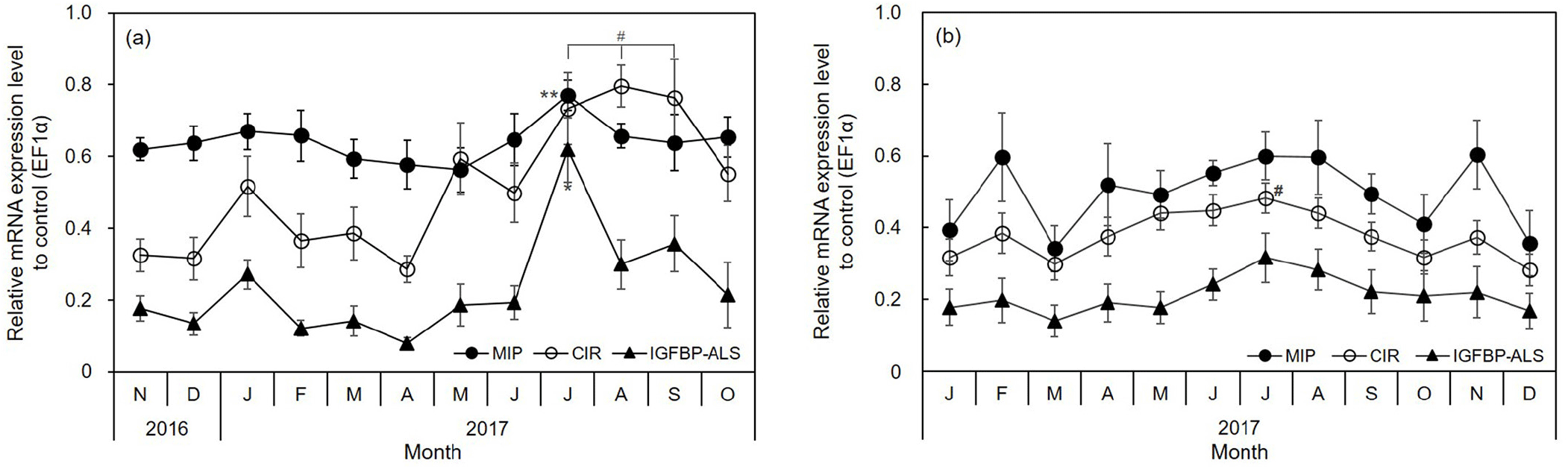
The molluscan insulin-related peptide (MIP), C. gigas insulin receptor-related receptor (CIR), and IGF binding protein complex acid labile subunit (IGFBP-ALS) mRNA expression varied monthly and seasonally in the adductor muscle. In the IGF system, the average expression in the adductor muscle was in the order MIP>CIR>IGFBP-ALS in both oyster groups. The expression of MIP and IGFBP-ALS was highest (Fig. 5) when the CI and TWR were the lowest (in July, Fig. 2). In particular, CIR expression was higher from July to September than during other months in diploid oysters (Fig. 4A). These genes were expressed more strongly in the warm summer and autumn months than in winter. In July, the expression of the three genes was significantly high (p<0.01). Moreover, the IGFBP-ALS levels increased by over 3.0-fold (steeply from 0.19 in June to 0.62 in July, Fig. 5A). There were no significant differences in triploid oysters, but the MIP, CIR, and IGFBP-ALS expression was high in July (Fig. 5B).
DISCUSSION
We investigated the correlations between IGF-related gene expression and protein levels in the adductor muscle of diploid and triploid oysters (C. gigas). There were differences in growth between diploids and triploids based on SH, SL, STW, and TW. Triploid oysters grow faster than diploid oysters because diploid oysters use more energy for gametogenesis (Allen & Downing, 1986; Nell & Perkins, 2005; Kim & Choi, 2019). In the present study, TWR and CI were significantly higher in triploid oysters during winter and spring except April (p<0.05), while in diploid oysters they were high only in May, just before the spawning season. Normand et al. (2008) reported a similar tendency. Moreover, in Hong Kong oysters, triploid oysters had a better biochemical composition and nutritional quality (Qin et al., 2018, 2019).
MHC and actin are components of thick and thin filaments, respectively. MHC is the motor protein of thick filaments and its isoforms are critical in determining functional variation among muscles (Wells et al., 1996). Actin activates muscle contraction by interacting with myosin to induce sliding of filaments (Millman, 1967; Funabara et al., 2013). We identified ACT2 as one of the actin isoforms, which is identical to ACT1 except for three residues near the N-terminus (Tanaka et al., 2018). In this study, the ACT2, MHC, and FIL-C levels in adductor muscle differed markedly in triploid oysters but not in diploid oysters (Fig. 4). In addition, the expression levels of ACT2, MHC, and FIL-C in diploid oysters were very similar and significantly highest in July (Fig. 4A). By contrast, those of triploids were not significant throughout the year, except ACT2 (Fig. 4B). This is in agreement with Tanaka et al. (2018) who found that actin and MHC expression in striated muscle of jellyfish varied according to developmental stage. Filamins are a family of actin-binding proteins comprising filamin A, B, and C in mammals (Van der Flier & Sonnenberg, 2001; Méndez-López et al., 2012). Filamins are involved in cell motility, cell signaling, transcription regulation, and organ development in vertebrates (Zhou et al., 2010). In humans, lack of filamins leads to malformation of the skeleton, brain, and heart (Zhou et al., 2010). Two different isoforms of filamin are present in muscle of the sea mussel (Mytilus galloprovincialis) one of which interacts with calponin-like protein (Méndez-López et al., 2012).
The levels of all genes identified decreased in December and increased in January, and then waxed and waned to April in diploid oysters. The gene levels were highest in May, concomitantly with the highest CI value, and rapidly declined in June, the start of the spawning season. Our data suggest that the gene expression pattern in the adductor muscle affects the growth of the Pacific oyster.
Previously, we reported that expression of the IGF system in C. gigas, in relation togrowth rate (Choi et al., 2018). MIP is involved in the control of body and shell growth in mollusks (Gricourt et al., 2003). CIR affects gonial proliferation and organogenesis (Gricourt et al., 2006) IGFBP-ALS functions in increasing the half-lives if IGFs in the bloodstream (Forbes et al., 2012). In this study, the MIP, CIR, and IGFBP-ALS mRNA expression in diploid oyster continuously increased during months of gonadal maturation and peaked in July before spawning in August and succeeding months. In triploid oyster, no major variation was observed throughout the year since triploid animals are often regarded as sterile or infertile (Dheilly et al., 2014). This means that these genes are involved during reproductive maturation and growth. The changes in the expression of the genes investigated in this study could be used as intrinsic indicators to determine the annual growth, maturity, and spawning period of cultured C. gigas in Korea.
In conclusion, the expression of three genes in the adductor muscle of C. gigas was correlated with the CI. The expression of all genes identified was higher in May, when the oyster is mature and about to undergo spawning, and exhibits greater valve activity and energy production in diploid oysters. However, they were not affected in triploid oysters. In addition, the expression of IGF-related genes was higher in July, when diploid oysters produce large numbers of spawn. However, the expression levels were similar to those in triploid oysters. Therefore, these changes in gene expression may be used as intrinsic indicators of the annual growth, maturity, and spawning period of cultured C. gigas using a suspended long-line culture system in Tongyeong, Korea. Further research should determine gene expression levels in other tissues, including the gonads, in connection with growth and maturity.

