INTRODUCTION
Mammalian puberty is initiated by the activation of hypothalamic gonadotropin-releasing hormone (GnRH) neurons (Ojeda & Skinner, 2006), which was induced by cooperation between increased excitatory input and decreased inhibitory input to hypothalamus (Bangalore Krishna & Witchel, 2024). Based on this, numerous studies have been conducted on the relationship between sexual maturity occurring during puberty and the increasing level of hormonal factors secreted by the hypothalamus, pituitary, and gonads, but the exact mechanism of puberty onset is still incomplete (Parent et al., 2003). Human GnRH is temporarily secreted from the fetal stage, and the secretion has a resting stage that weakens over the next few years. The GnRH signal is dynamically reproduced again at the time of sexual maturity after the resting stage, and this is depending on the KiSS-1 gene product, kisspeptin, and its receptor, GPR54 (Seminara, 2003; Plant & Barker-Gibb, 2004). The cell bodies of the kisspeptin neurons are present in the hypothalamic arcuate nucleus (ARC) and the anteroventricular nucleus (AVPVN) (Gottsch et al., 2004; Kinoshita et al., 2005), and ARC is known as a region that produces the pulsatile GnRH secretion (Maeda et al., 2007). Signaling formed by kisspeptin and its receptor GPR54, is known to stimulate the secretion of GnRH neurons and gonadotropic hormones (de Roux et al., 2003; Irwig et al., 2005). In addition, kisspeptin-GPR54 signaling is considered as a key factor in sexual maturity, playing an important role in the transition to puberty (Gianetti & Seminara, 2008). Studies have shown that kisspeptin neurons in ARC are also known to produce neurokinin B (NKB) (Navarro et al., 2009; Koysombat et al., 2025). NKB and its receptor neurokinin 3 receptor (NK3R) are also presumed to be involved in sexual maturity in puberty (Navarro & Tena-Sempere, 2011). In mammals, NKB-NK3R signals seem to regulate reproductive function at least in part through the regulation of activity of kisspeptin neurons (Wakabayashi et al., 2010).
Interestingly, presence of gonadotropin-inhibitory hormone (GnIH) that inhibits the secretion of gonadotropins from the pituitary gland by counteracting kisspeptin in birds was reported (Tsutsui et al., 2000). Although it has been questioned whether the GnIH found in birds and later in mammals has the opposite function to GnRH (Kriegsfeld et al., 2006), evidence indicates that mammalian GnRH secretion could be dynamically controlled by the interaction between GnIH and kisspeptin (Ebling & Luckman, 2008; Smith et al., 2008). Probably, the activation of hypothalamic GnRH neurons might be induced by cooperation with the excitatory-inhibitory signal of kisspeptin-GnIH in the onset of puberty (Parent et al., 2003).
Since the physiological natures of the factors and their regulatory mechanisms that determine the mammalian puberty onset are crucial to understand the reproductive problems associated with puberty such as precocious and/or delayed puberty, we investigated the temporal changes in activity of reproductive hormone-related genes in the hypothalamus-pituitary (HP) axis as a first step in the present study.
MATERIALS AND METHODS
Sprague-Dawley rats were provided by DBL (Eumseong, Korea) and reared in Sangmyung University animal facility under photoperiods of 12 h light/dark with lights on at 7 AM and constant temperature of 21°C–23°C. Food and tap water were supplied ad libitum. The animal protocols were approved by the Animal Care and Use Committees at Sangmyung University (Approval code R-1104). All the animals received human care in accordance with the guides for animal experiments of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
The vaginal opening (VO) was visually confirmed, and the vaginal epithelium of the open animals was collected by smear using physiological saline. The epithelial cells were applied to microscope slides, fixed with cold acetone (Merck, Darmstadt, Germany), stained with Eosin (Sigma-Aldrich, St. Louis, MO, USA) and observed.
Animals were sacrificed at 2-day intervals between postnatal day (PND) 29 and PND 43 days. The trunk bloods were immediately transferred to a plastic test tube and centrifuged at 3,000×g for 15 minutes, and the sera were separated and stored at –80°C until the hormone assay. Meanwhile, major tissues were collected and weighed immediately after the sacrifice (Metler-Toledo, Greifensee, Switzerland).
Quantification of the levels of serum estradiol (E2) and progesterone (P4) were measured using radioimmunoassay with γ counter system (Cobra II, Packard, Downer Grove, IL, USA). Steroid hormones in serum were extracted using diethyl ether (Merck) and dissolved in gelatin phosphate-buffered saline. Quantification of E2 was performed using a Coat-A-Count Estradiol kit [Domestic Policy Council (DPC), Washington, DC, USA], and the measurable sensitivity was 8 pg/mL. Intra-assay coefficient variation was 5.8±5.5%, and the inter-assay coefficient variation was 7.4±6.7%. Quantification of P4 was performed using the Coat-A-Count Progesterone kit (DPC), with measurable sensitivity of 0.02 ng/mL, intra-assay coefficient variation of 3.6±0.12%, and inter-assay coefficient variation of 3.9±0.13%.
Fixed tissues were dehydrated in graded concentrations of ethanol (70%, 80%, 90%, 95%, and 100%; Duksan, Ansan, Korea) for 1 h 30 min in each with gentle shaking and soaked in absolute ethanol overnight. The tissues were immersed in xylene (Samchun Chemical, Seoul, Korea) for 30 min, 3 times and in paraffin at 56°C for 30 min, 3 times. The tissues were embedded in paraffin and sectioned (Microm, Walldorf, Germany) at 5 μm. The samples were attached on microscope slides and the slides were stained with hematoxylin (Sigma-Aldrich) for 5 min and eosin (Across, Carson, CA, USA) for 5 min, respectively.
Total RNAs were used in RT-PCR carried out with Maxime™ RT PreMix (InTron, Seongnam, Korea) and AccuPower PCR Premix (GeneAll, Seoul, Korea) according to the manufacturer’s instructions. Sequences of the primers and the specific PCR conditions used in this study were successfully worked in our pilot study, and were listed in Tables 1 and 2, respectively. The reactions were subjected to MultiGene™ OptiMax Thermal Cycler (Labnet, Edison, NJ, USA). The reaction products were analyzed by gel electrophoresis in 1.5% agarose gel (75 V, 65 min) and visualized by ethidium bromide staining. The band intensities were measured using the image analysis system (Imager Ⅲ-1D main software, Bioneer, Daejeo, Korea). Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as reference gene for normalization of quantitative RT-PCRs in the present study.
The directions of sequences are all 5’ to 3’.
PCR, polymerase chain reactions; F, forward; R, reverse; GnRH, gonadotropin-releasing hormone; KiSS-1, kisspeptin; GPR54, G protein-coupled receptor 54; GnIH, gonadotropin-inhibitory hormone; GPR147, G protein-coupled receptor 147; Tac, tachykinin; TacR3, tachykinin receptor 3; GAPDH, glyceraldehydes-3-phosphate dehydrogenase.
The directions of sequences are all 5’ to 3’
GnRH, gonadotropin-releasing hormone; KiSS-1, kisspeptin; GPR54, G protein-coupled receptor 54; GnIH, gonadotropin-inhibitory hormone; GPR147, G protein-coupled receptor 147; Tac, tachykinin; TacR3, tachykinin receptor 3; GAPDH, glyceralde- hydes-3-phosphate dehydrogenase.
All experiments were performed at least three times. Values were expressed as mean±SE. Data were analyzed using Student’s t-test and/or one-way analysis of variance (ANOVA) as indicated. p<0.05 was considered as statistical significance. Calculations were performed using Graphpad Software Prism version 6 (GraphPad Softward, San Diego, CA, USA).
RESULTS
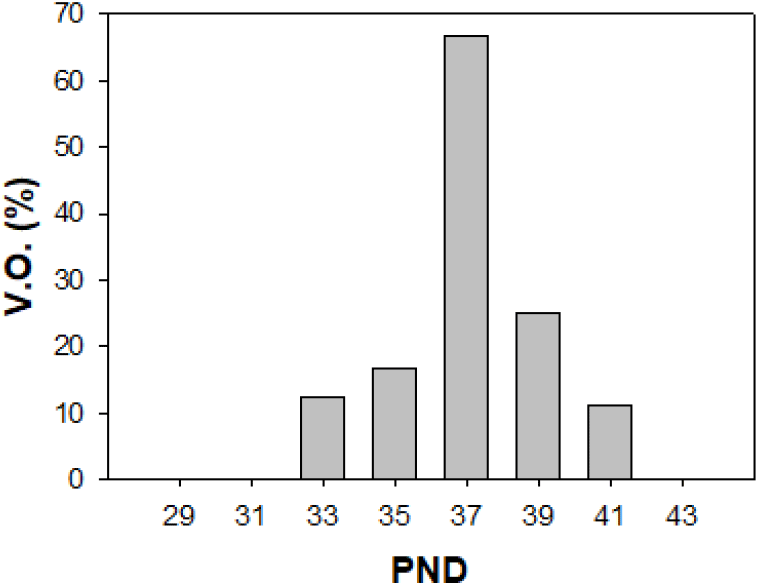
Immature female rats were examined for VO, an indicator of the onset of puberty, and sacrificed at intervals of 2 days from PND 29 to PND 43. The body weight gradually increased, which was increased significantly from PND 33 (p<0.01, Table 3). The weight of the ovaries, uteri and pituitary glands increased significantly from PND 37 (p<0.01), while the weight of the oviducts showed significant increase from the PND 39 (p<0.01). The rates of VO were found to be the highest at PND 37 (66%, Fig. 1).
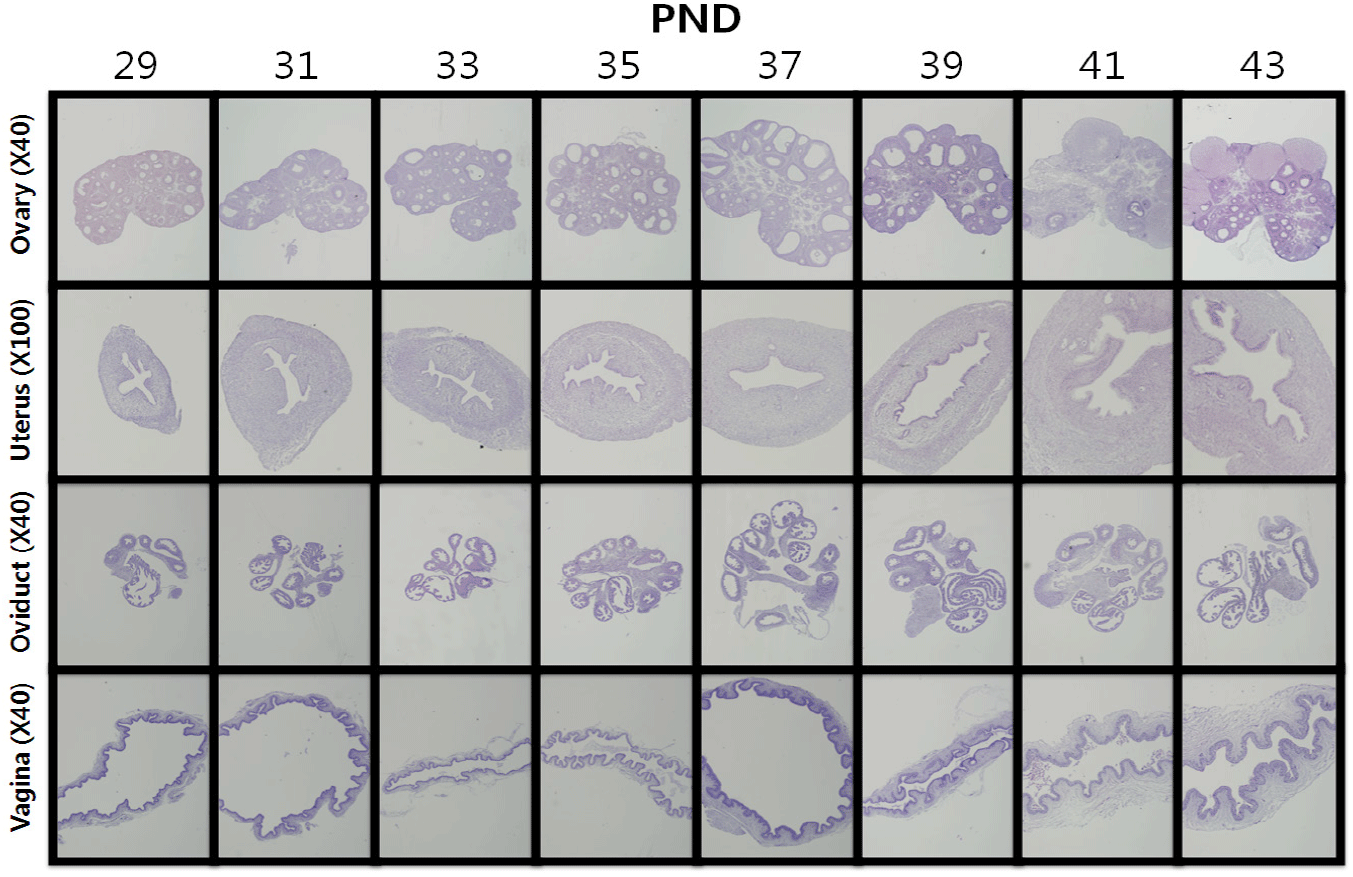
Histological investigation (Fig. 2) showed that small primary and secondary follicles and degenerating follicles were mainly observed in the ovary from PNDs 29, 31, and 33. A number of Graafian follicles and corpus luteum, indicators of sexual maturity, were observed from PND 37. The uterus also gradually matured, and the luminal epithelia and myometrium developed thickly, and a number of endometrial glands were identified on PNDs 39 and 41. The oviduct also gradually matured, confirming that the lumen developed after PND 37.
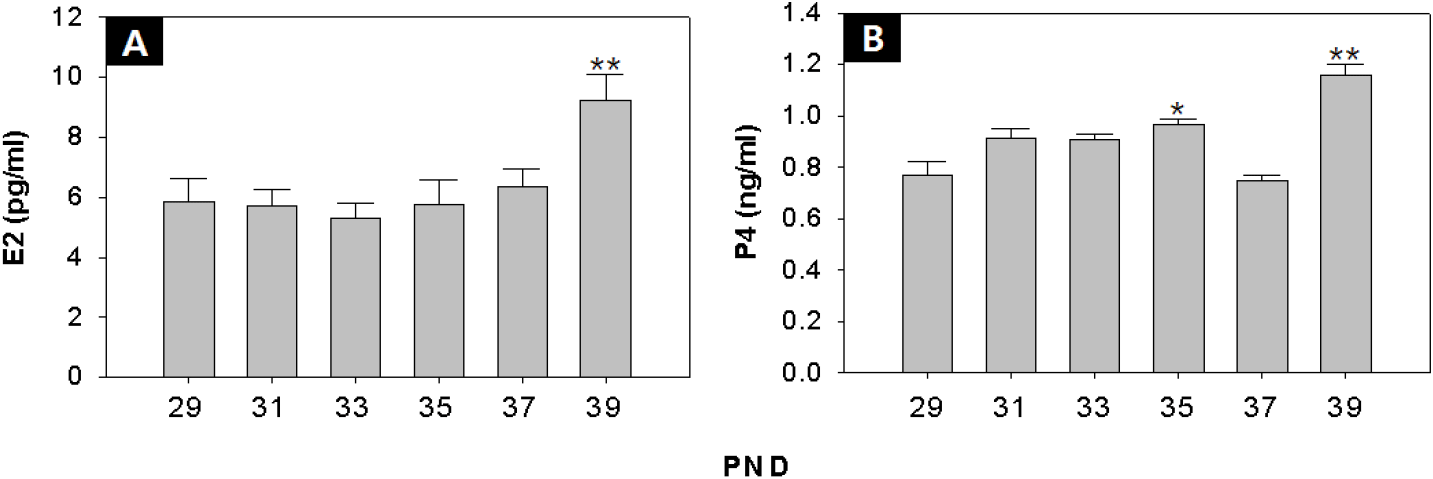
As a result of RIAs, there was no significant change in serum E2 levels from PND 29 to PND 37, but it increased significantly at PND 39 (PND29:39=5.9±0.7:9.2±0.9 pg/mL p<0.01) (Fig. 3A), and level of serum P4 showed an increase at PND 35 (PND 29:35=0.77±0.05:0.97±0.02 ng/mL p<0.05) (Fig. 3B).
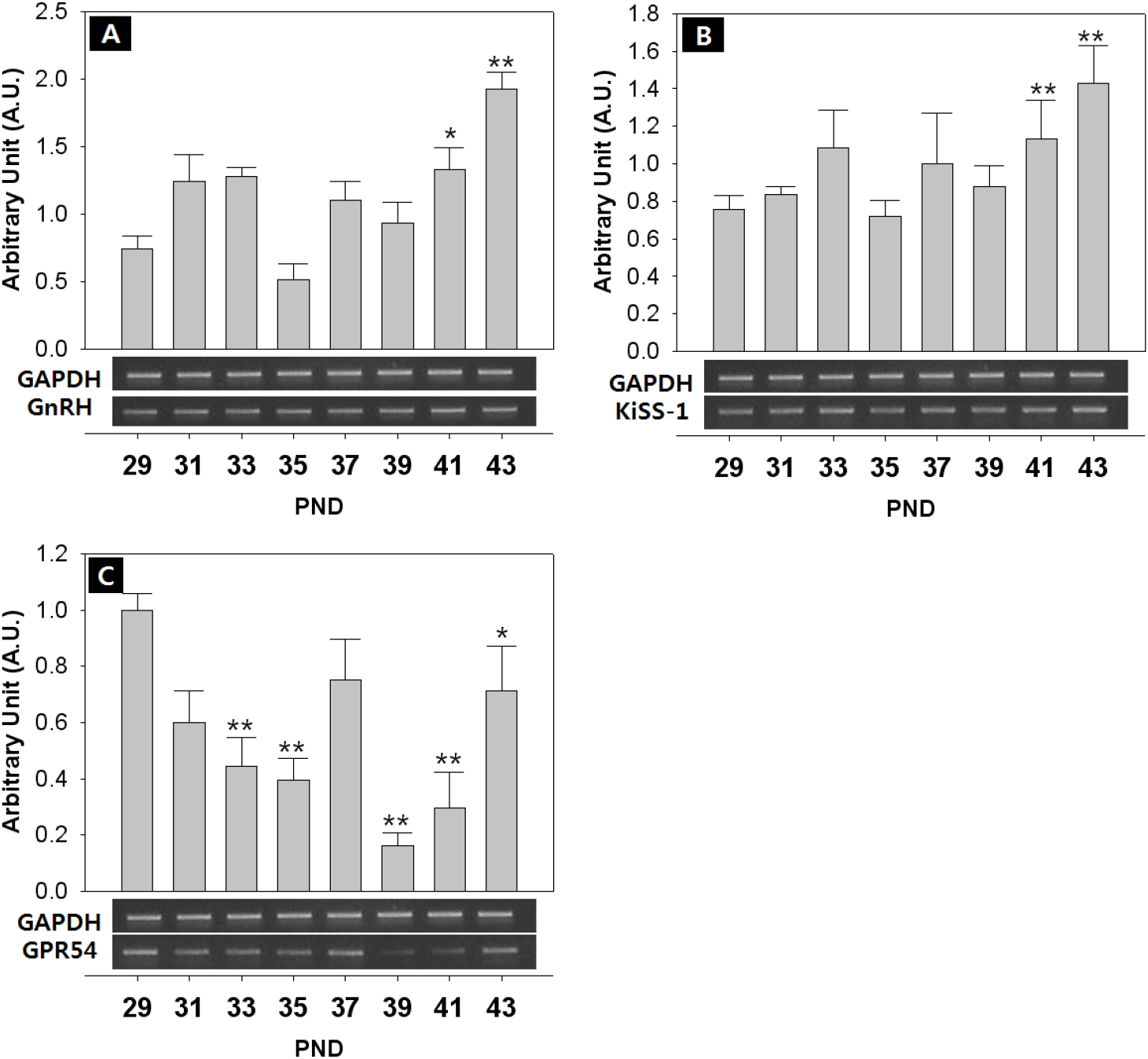
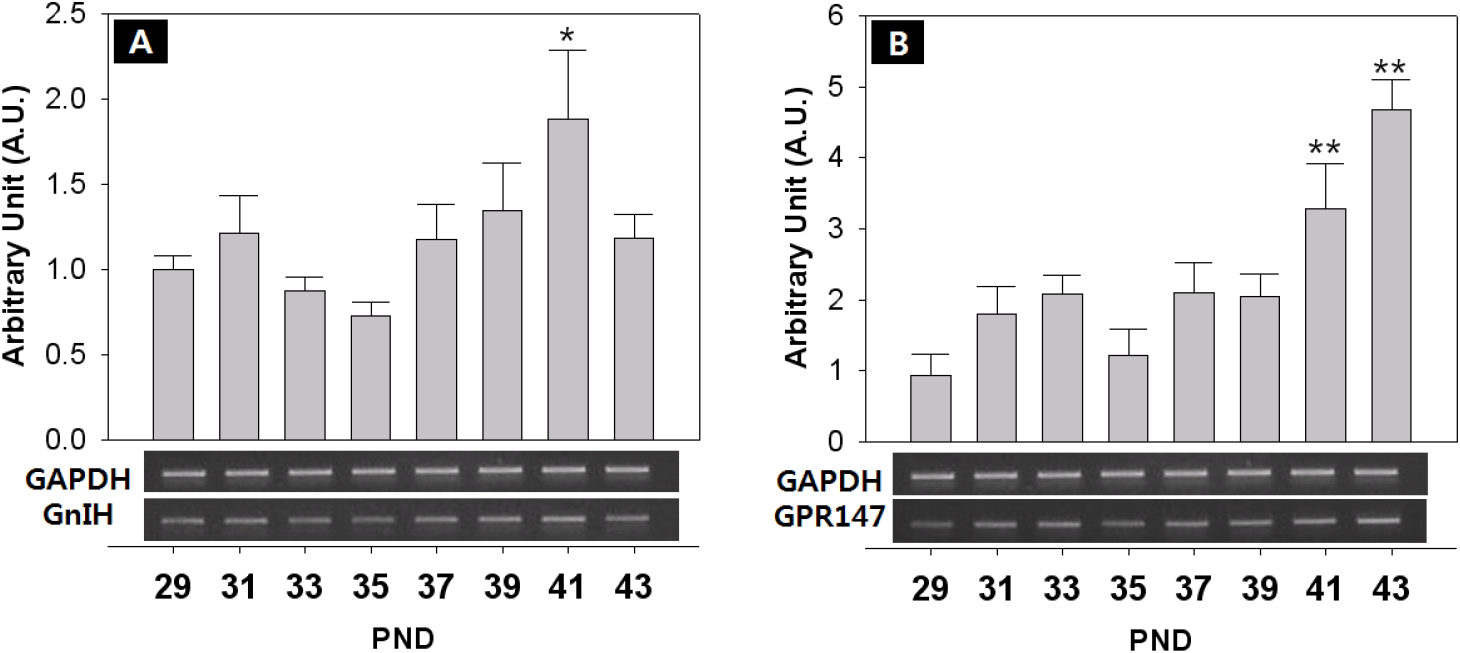
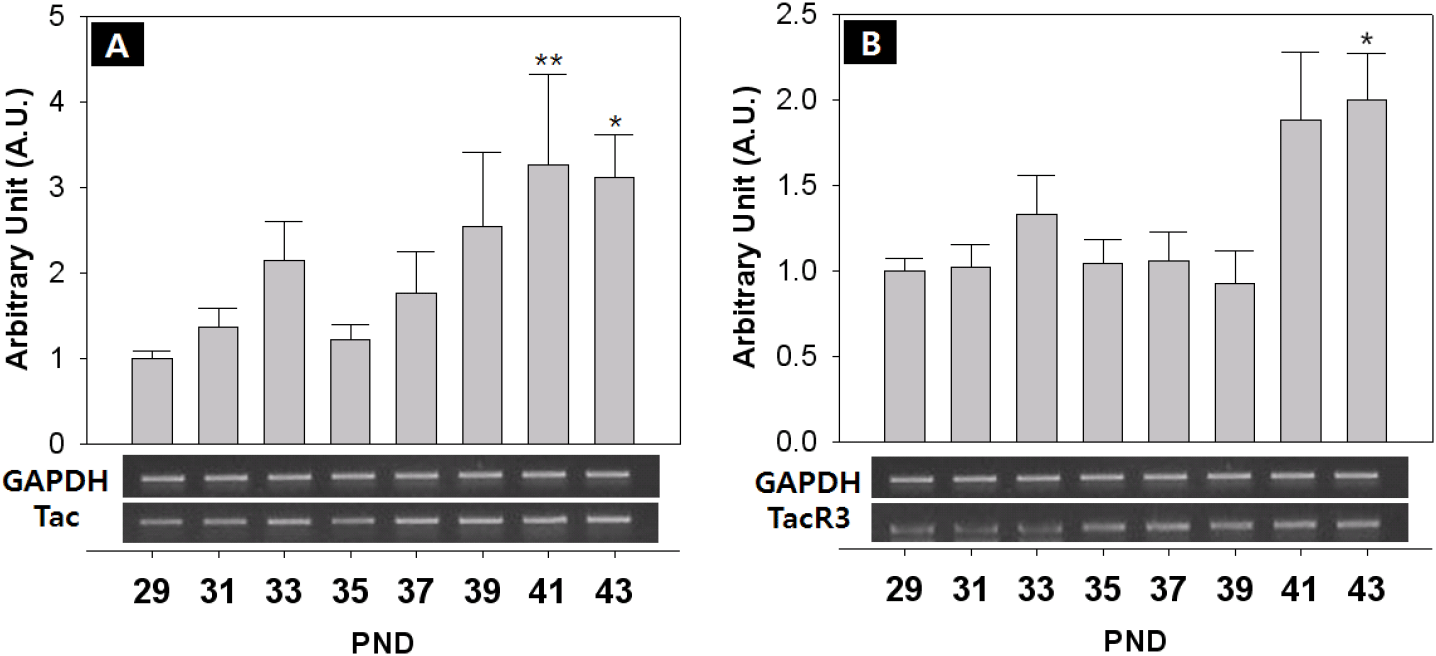
The expression of KiSS-1/GPR54 that is presumed to promote and regulate the initiation of puberty, was investigated through RT-PCR. The expression of GnIH/GPR147 that is known to inhibit the expression of LH and FSH in contrast to GnRH, was also investigated. Finally, the expression of NKB and its receptor that has recently been reported to be involved in the regulation of the puberty onset was investigated. Generally, all the expressions showed fluctuated pattern as the day progressed. The level of GnRH mRNA showed an increase from PND 29, but decreased slightly from PND 35, and increased significantly at PND 41 (PND29:41=0.74±0.09:1.33±0.16 AU p<0.05) and PND 43 (PND29:43=0.74±0.09:1.92±0.12 AU p<0.01) (Fig. 4A). The expression of KiSS-1 also showed a similar pattern to that of GnRH on PND 41 (PND29:41=0.75±0.08:1.13±0.20 AU p<0.01) and PND 43 (PND29:43=0.75±0.08:1.43±0.20 AU p<0.01). (Fig. 4B). In addition, the expression of GPR54 significantly decreased from PND 29 to PND 35 (PND29:35=1.00±0.06:0.40±0.10 AU p<0.01), and then significantly increased from PND 39 until PND 43 (p<0.01, Fig. 4C), showing a difference from the expression patterns of GnRH and Kisspeptin. GnIH doubled at PND 41 (PND29:41=1.00±0.08:1.72±0.08 AU p<0.05) (Fig. 5A), and GPR147 showed pulsatile pattern from PND 29, and significantly increased at PND 41 (PND29:41=1.00±0.08:1.89±0.41 AU p<0.01) and PND 43 (PND29:43=1.00±0.08:1.18±0.14 AU p<0.01). (Fig. 5B). Finally, expression of NKB (Tac) significantly increased on PND 41 (PND29:41=1.00±0.08:3.26±1.07 AU p<0.01) and PND 43 (PND29:43=1.00±0.08:3.11±0.51 AU p<0.05) (Fig. 6A), and expression of TacR3 showed a similar pattern to Tac, increased at PND 43 (PND29:43=1.00±0.07:2.00±0.27 AU p<0.05) (Fig. 6B).
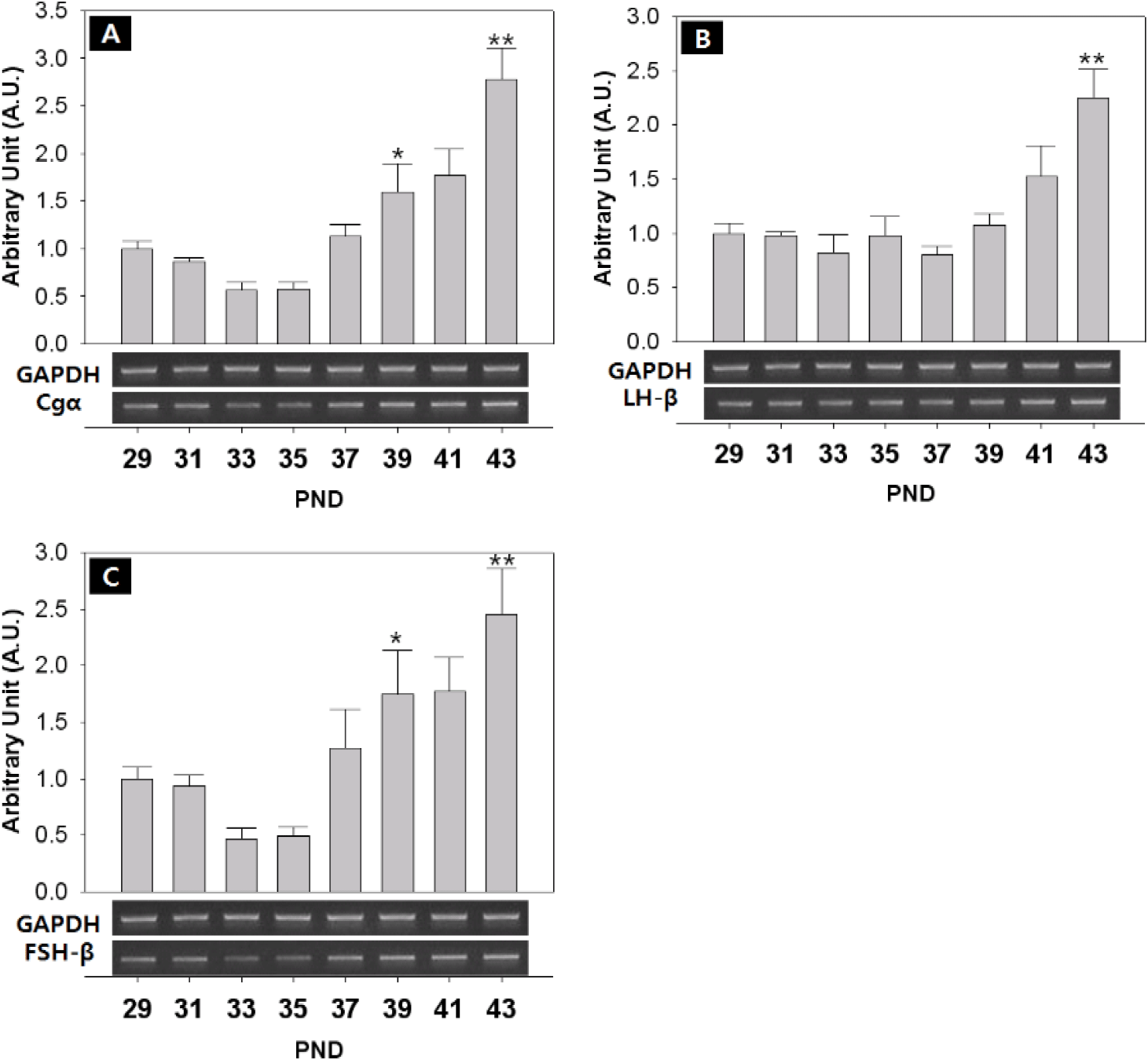
The mRNA level of pituitary glycoprotein hormone alpha subunit (Cgα) significantly increased at PND 39 (PND29:39=1.00±0.08:1.60±0.30 AU p<0.05) and PND 43 (PND29:43=1.00±0.08:2.77±0.33 AU p<0.01) (Fig. 7A). The mRNA level of LH-β increased significantly at PND 43 (PND29:43=1.00±0.10:2.25±0.26 AU p<0.01). (Fig. 7B). The expression of FSH-β was similar to those of Cgα, significantly increased at PND 37 (PNA29:37=1.00±0.10:1.27±0.34 AU p<0.05) and PND 43 (PND29:43=1.00±0.10:2.46±0.40 AU p<0.01) (Fig. 7C).
DISCUSSION
The present study shows the rapid growth and maturation of reproductive organs immediately after VO, and dynamic changes in gene expression of the HP axis in peripubertal female rat. Expression profiles of known stimulatory and inhibitory signals (kisspeptin and GnIH, respectively) on the hypothalamic GnRH pulse generator had no prominent aspect. Interestingly, the expression profile of kisspeptin is very similar to that of GnRH, indicating direct connection of two neurons (Oakley et al., 2009). Generally speaking, our PCR results showed that most of the expression of hypothalamus and pituitary factors tended to increase after VO, and the patterns were rather unstable and no significant peak pattern such as LH surge shown in proestrus adults even in LH subunits expression.
According to the previous report the average VO day of SD strain rat is PND 35 (Rasier et al., 2006), and the first ovulation occurs within 5 days after VO (Urbanski & Ojeda, 1985). In the present study, date of VO was confirmed in 66% of all experimental animals at PND 37. The reason why VO was delayed by 2 days compared to the past report is due to the use of experimental animals born between November and January in the present study. It has been reported that puberty is delayed in low-temperature environments (Drickamer, 1990), and the animals born between December and February were delayed by about three days compared to those born between April and August in an environment where the light:dark cycle was set at 14:10 hours, and in other light:dark cycle animals born in January were also delayed by about three days compared to those born in August (Cohen & Mann, 1979).
In the present study, the body weight increased significantly from PND 33, and the weight of reproductive tissues increased significantly after PND 37, which is just over the average VO. The initiation of puberty begins with the activation of GnRH neurons (Ojeda & Skinner, 2006), and LH and FSH are secreted from the pituitary by GnRH, inducing follicle development and ovulation in female reproductive organs (Brown & McNeilly, 1999). In rats, the uterine weight gains are occurred from the PND 21, meaning that the activation of the estrogen system begins (Hany et al., 1999). In the present study, the pubertal weight gains of reproductive organs seem to happen simultaneously with the activation of hypothalamus-pituitary-ovary (HPO) hormonal axis. E2 induces the growth and development of follicles (Goldenberg et al., 1972), and regulates P4 action by activating the expression of progesterone receptors (PRs) (Kastner et al., 1990). In a study comparing serum levels of E2 and P4 at PND 33 just before the onset of puberty and at PND 53 after puberty, there was no significant difference in E2 level between PND 33 and PND 53 while 5 times higher in P4 level at PND 53 compared to level at PND 33 (Grote et al., 2006). In the present study, RIA revealed that both E2 and P4 levels showed a significant increase on PND 39 (p<0.01, respectively) immediately after the onset of puberty. Another report showed that kisspeptin-treated rats had delayed pubertal onset and reduced feed intake and body weight, suggests that kisspeptin may act as a mediator between reproductive and metabolic phenomena in rodents (Sathagopam et al., 2021).
Hypothalamic GnRH exhibits pulsatile secretion patterns, causes the secretion of gonadotropic hormones in the pituitary gland, and causes sex hormone secretion and gametogenesis in gonads (Conn & Crowley, 1994; Pfaff et al., 1994; Millar et al., 2004). In order for the puberty onset to take place normally, integrated regulation of various central and peripheral inputs, including the GnRH pulse generator in the hypothalamus, is required. The increased GnRH secretion in puberty is initiated and maintained by changes in the intersynaptic input and glial cell input into GnRH neurons (Guarraci et al., 2022; Ojeda & Terasawa, 2002; Ojeda et al., 2010). In 2003, mutation(s) of KiSS-1 and its receptor GPR54 was discovered in patients with thrombocytopenic hypogonadotropic hypogonadism, suggesting that kisspeptin-GPR54 signaling seems to be closely related to reproductive processes such as adolescent initiation and sexual maturity (de Roux et al., 2003; Seminara et al., 2003; Dungan et al., 2006; Roa et al., 2008; Oakley et al., 2009). When immature female rats were administered kisspeptin peptide through the intracerebroventricular (icv) injection from PND 26 to PND 31, the time to taken to initiate VO was shortened, the uterine weight was increased, and the serum levels of LH and E2 were increased compared to the control group. In addition, hypothalamic KiSS-1-GPR54 were expressed at the highest levels during puberty (Navarro et al., 2004). The hypothalamic kisspeptin system is thought to play a role in transmitting promotional signals to GnRH neurons (Castellano et al., 2010). Indeed, kisspeptin-GPR54 signals play an important role in the regulation of the HPO axis in the hypothalamic neural circuit, and are closely related to the induction of GnRH pulse which increases during puberty. In our results expression profiles of GnRH and KiSS-1 showed a significantly increasing pattern after the VO, confirming that the kisspeptin-GPR54 signal plays a role in advancing sexual maturity with an increase in GnRH secretion.
Mutations in Tac and TacR3 were discovered in patients with hypogonadism, and it has been reported that NKB and NK3R play an important role in the regulation of reproductive function (Topaloglu et al., 2009). NKB is expressed in KiSS-1 neurons at ARC that produce GnRH pulse (Navarro & Tena-Sempere, 2011), and it has been reported that administration of NK3R antagonists in the period before and after puberty in female rats tends to delay the timing of VO and reduce LH levels (Navarro et al., 2012). In the present study, the expressions of Tac and TacR3 similarly increased after the VO day. As Tac and TacR3 increase after the puberty onset, the Tac-TacR3 system seems to be highly related to sexual maturity.
Until the discovery of GnIH in avian brain, it was unclear about the presence of neuropeptide factors that inhibit the secretion of gonadotropic hormone (Tsutsui et al., 2000; Osugi et al., 2004). In mammals, cDNA encoding RFamide peptide (RFRP-1) similar to GnIH in birds was discovered through genetic database search (Hinuma et al., 2000). It has since been reported that RFRP-1 plays a potential role in the regulation of prolactin secretion in the pituitary gland in mammals (Yoshida et al., 2003), and that RFRP-3 directly inhibits GnRH neurons in rodents (Ducret et al., 2009). As GnRH secretion was dynamically controlled by the interaction between GnIH and kisspeptin (Smith et al., 2008), GnIH in the GnRH neuronal circuit is presumed to act as an inhibitory signal against kisspeptin at the onset of puberty (Parent et al., 2003). Unexpectedly, our RT-PCR showed that GnIH-GPR147 expression profiles were similar to GnRH expression profile, not an opposite way.
LH and FSH secreted from the pituitary gland are synthesized and secreted from glycoprotein hormone-secreting cells in the anterior pituitary gland. LH and FSH act to stimulate follicle formation and ovulation in females (Brown & McNeilly, 1999). LH and FSH belong to the glycoprotein hormone family together with the pituitary hormone thyroid-stimulating hormone (TSH) and placental chorionic gonadotropin (CG), and are composed of two subunits α and β. In these dimeric hormones, α-subunits share in common, and their biological properties are determined by β-subunits those have the same characteristics of each hormone (Pierce & Parsons, 1981; Gharib et al., 1990). Our RT-PCR study demonstrated that Cgα significantly increased from PND 43. In the case of FSH-β, like Cgα, increased at PNDs 39 and 43. The increase in Cgα, LH-β, and FSH-β all after the onset of puberty is due to the increase in GnRH expression after the onset of puberty, and it is shown that the HPO axis is activated with the onset of puberty.
The most important factor controlling puberty in relation to eating was Leptin (Buchanan et al., 1998; Cunningham et al., 1999), and Leptin increases the secretion of gonadotropins and increases the weight of the reproductive organs (Barash et al., 1996). There is an evidence that precocious puberty in humans may be related to obesity in adolescents (Ebling, 2005). In female rats, a study reported the VO period was shortened by about 5 days in the experimental group fed with high-fat diet (Lee et al., 2009). In addition, endocrine disruptors (EDCs) are known to delay or accelerate the onset of puberty (Colborn et al., 1993), and EDCs affect the development and reproduction of various animals, including humans, and can act directly on sex hormone receptors or affect the synthesis and metabolism of transcription factors (Toppari & Skakebek 1998; Takeyoshi et al., 2002). These effects can also affect the HPO axis, which is closely involved in the regulation of sexual maturation (Toppari, 2002). Abnormal adolescent initiation due to these factors can interfere with normal sexual maturation or cause a decrease in pregnancy rates in adulthood (Bates et al., 1982; Anderson, 1980).
The present study is the first step in identifying regulating factors with sequential expression pattern and puberty initiation. As a result of the study, it was confirmed that various signaling systems involved in the HP axis dynamically changed in female rats peripubertal period. These dynamic changes are thought to reflect the imperfections in the state of reproductive endocrine at the onset of puberty. In order to achieve a normal initiation of puberty, the activation of GnRH neurons and the dynamic interaction between the KiSS-1/GPR54 signaling and the GnIH/GPR147 signaling seem to be crucial.
The fundamental identification of the mechanism of puberty initiation will be of great help for understanding reproduction. Furthermore, it could be able to provide a clue to medical and veterinary coping with abnormal initiation of puberty and accompanying abnormal symptoms (e.g., obesity).

