INTRODUCTION
Epinephelinae are known to be protogynous hermaphrodites in that they are all born female, and at some point in their lifespan some change sex to male. The grouper’s natural sex reversal has been found in the greasy grouper E. tauvina (Chao & Lim, 1991) showing it took seven years to have a natural sex reversal after hatching. Another study on the goldblotch grouper E. coatae (Glamuzina et al., 2000) showed 11 kg body weight fish naturally reverse sex. As for the redspotted grouper E. akaara (Hamamoto et al., 1986), the fish longer than 25 cm total length and heavier than 500 g body weight, had an increased ratio of males. Sex reversal in groupers makes it difficult to secure male fish in wild and seed production. Thus, sex reversal hormone treatment is being used to produce functional males by injecting hormones into young female grouper. Previous experiments attempted masculization in groupers through hormonal manipulation. In the greasy grouper E. tauvina (Chen et al., 1977; Chao & Chow, 1990), sevenband grouper E. septemfasciatus (Tsuchihashi et al., 2003; Song, 2004), and redspotted grouper E. akaara (Hwang et al., 1998), longtooth grouper E. bruneus (Oh et al., 2003), functional males were obtained when endogenous androgen or 17α-methyltestosterone (MT) was administered. In the honeycomb grouper E. merra (Alam et al., 2006), an aromatize inhibitor, or an endogenous hormone, was used to induce masculinization.
Sperm storage, or sperm cryopreservation, is being used to preserve sperm cells to account for difficulty in securing male fish, inconsistent sexual maturation age and size, and gender imbalance that make it difficult to get sperm at the optimal timing for seed production. Sperm cryopreservation has the benefit in that sperm from healthy fish can be selected to prevent disease. Also, it is economically more efficient than using larvae for breeding (Lubzens et al., 1997). It has been used in many species such as: the tilapia Sarotherodon mossambicus (Harvery, 1983), the common carp Cyprinus carpio (Kurokura et al., 1984), the rainbow trout Salmo gairdneri (Baynes & Scott, 1987) in freshwater fish and the alabar grouper Epinephelus malabaricus (Chao et al., 1992), the tiger pufferfish Takifugu rubripes (Chang et al., 1998), the black sea bream Acanthopagrus schlegeli (Lim et al., 1997), and the flatfish Paralichthys olivaceus (Zhang et al., 2003) in seawater fish. This study aims was to evaluate of fertilizing ability using frozen thawed sperm to investigate the fertilization rate and the hatching rate depending on the cryoprotectant concentrations for cryopreservation and the cryopreserved period to find optimal sperm levels and better preservation techniques for artificial seed production of longtoogh groupers.
MATERIALS AND METHODS
The semen for the experiments was collected from a wild caught longtooth grouper (total length 76 cm, body weight 6.4 kg) that had been reared in an inner concrete tank (5×5×3 m), with the water capacity of 62 m3 at the Ocean and Fisheries Research Institute in Jeju Province in 2005.
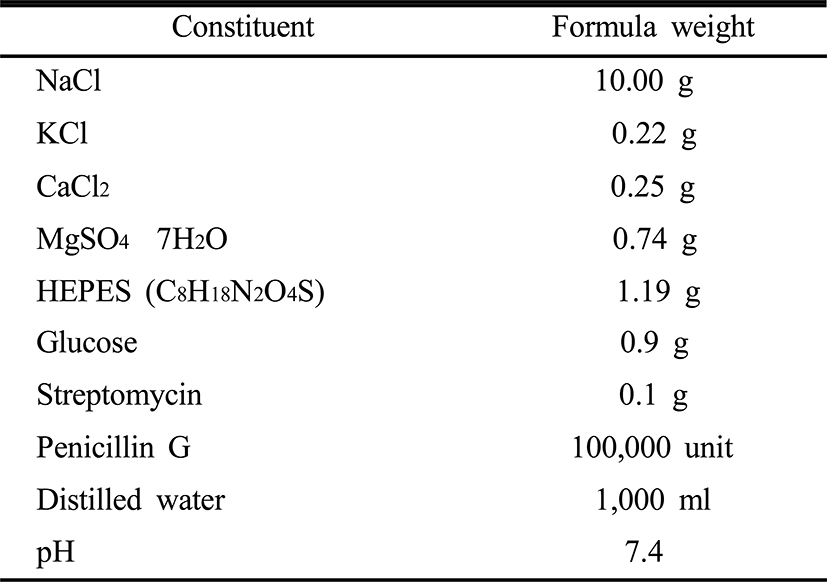
For sperm cryopreservation in longtooth groupers, Ringer’s solution was used as a diluent (Table 1) and dimethyl sulfoxide (DMSO, Sigma Co., Ltd. USA) was used as a cryoprotectant. The equilibration time between a semen and the diluent was regulated within 1 minute, and a 0.5 ml semen straw was used for freezing.
|
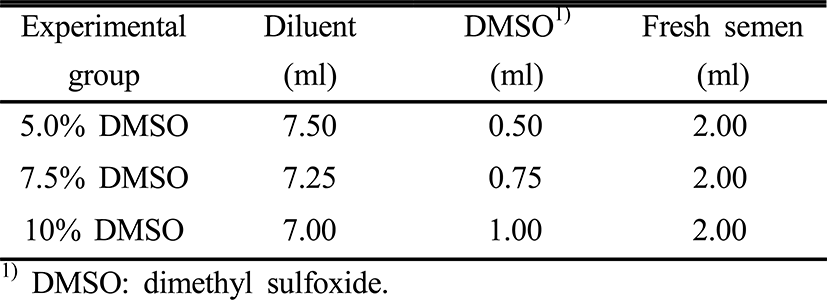
To determine the optimal proportion of diluents and cryoprotectants that minimizes freezing injuries during the procedure to cryopreserve the sperm, diluents and cryoprotectants were mixed in different proportions (Table 2). We placed 0.5 ml of the diluted semen with cryo protectants into the straw and then sealed the straw. The sealed straw was stored in a liquid nitrogen tank (–196°C) after being frozen for 15 minutes at liquid nitrogen vapor (–96°C). After 48 hours, the cryopreserved sperm were thawed in filtered seawater (22±1.0°C) and then used for the experiments. For the fertilization rates analysis depending on the DMSO concentrations, we mixed 20 g of the ovulated eggs administrating human chorionic gonasotropin (HCG, Calbiochem Co., Ltd. USA) and 0.1 ml of cryopreserved semen respectively and then added filtered seawater gradually to induce artificial fertilization. For the control group, cryoprotected fresh semen and diluents were diluted at the ratio of 1 to 49. 4 hours after the artificial fertilization, aliquots of the buoyant eggs were randomly selected and normally developing eggs were counted in a profile projector (PJ-H3000F, Japan) for the fertilization rates. For the hatching rates analysis depending on the DMSO concentrations, 80 to 100 fertilized buoyant eggs were randomly selected, counted under the profile projectors, placed in a 500 ml beaker, and then hatched. The hatching rates were calculated for the non fertilized eggs for the past 60 hours after the artificial fertilization whilst the water temperature was maintained at 21.0±0.5°C. The ratio of normally developed fish out of the total hatching larvae, excluding the ratio of dead fish right after the hatching and abnormally developed ones like those with spinal curvature, was calculated and every observation was done thrice repetitively.
|
The fertilization rates and hatching rates were analyzed to examine the fertilizing ability of the cryopreserved sperm according to the period of cryopreservation. We diluted DMSO and diluents at the ratio of 1 to 7, mixed DMSO and diluents with the ratio of 1 to 7, and then diluted the mixed solution with semen 5 times (semen 1: mixed solution 4). We then injected 0.5 ml of the diluted solution containing cryoprotectants into the straw and sealed the end of the straw. The sealed straw was stored in a liquid nitrogen tank after being frozen for 15 minutes at liquid nitrogen vapor. The fertilizing ability after being thawed was determined by fertilization rates and the hatching rates of the artificially fertilized embryos using thawed sperm after the cryopreservation 2 days, 2 years, and 3 years. The fertilization rates and hatching rates were analyzed in the same method as cryopreservation analysis depending on the DMSO concentrations.
RESULTS
The fertilization rates at different DMSO concentration groups of 5.0%, 7.5%, and 10.0%, respectively, were 99.5±0.8%, 99.5±0.7% and 99.6±0.6% showing the exact same results from the fertilization rates of the control group (P>0.05, Fig. 1A).
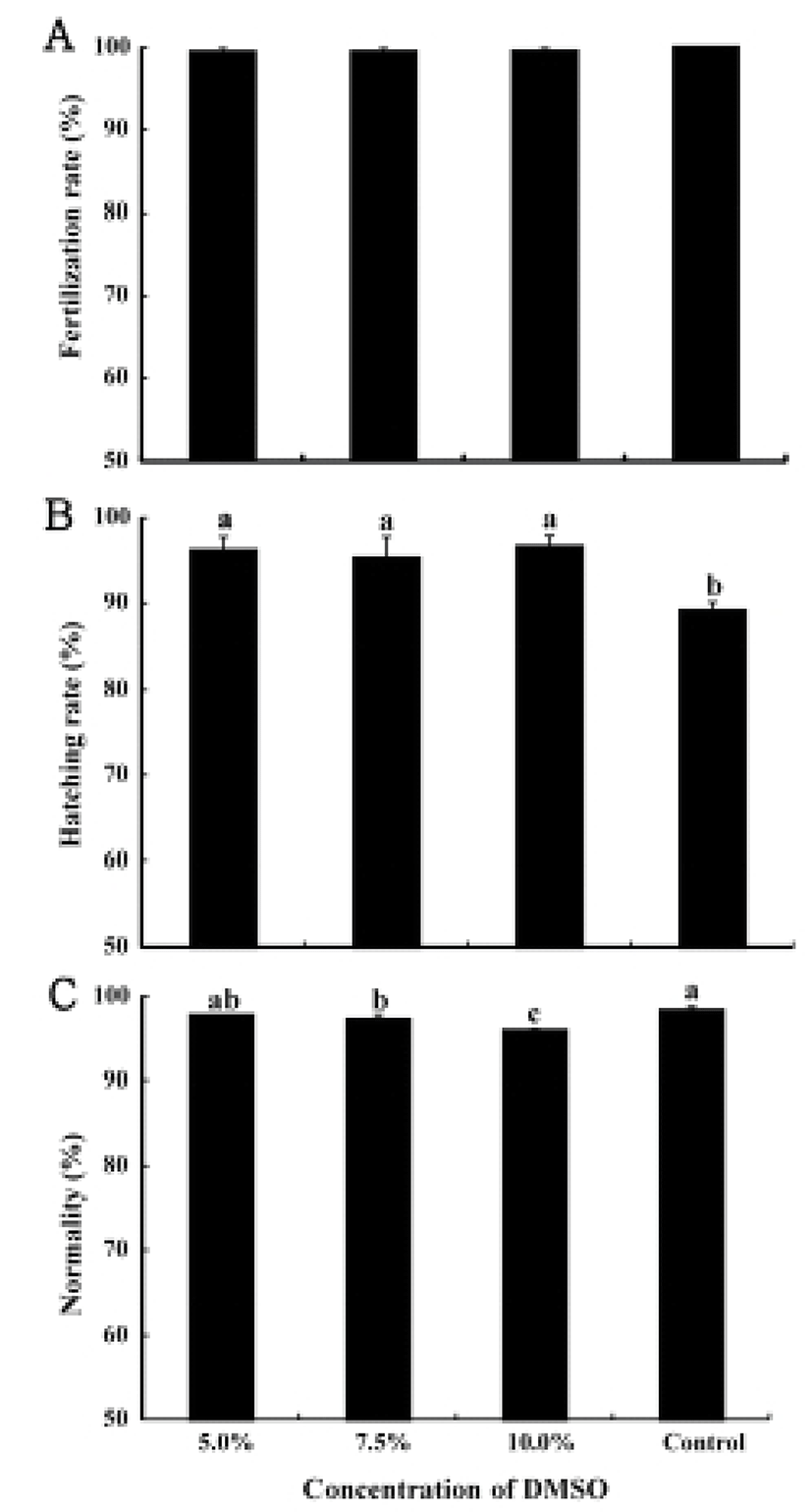
The hatching rates at different DMSO concentrations in control group were the lowest at 89.1±1.4% (P<0.05), and the hatching rates in the experimental groups of different DMSO concentrations of 5.0%, 7.5%, and 10.0% were 96.2±2.3%, 95.3±3.6%, and 96.6±1.8% respectively (P>0.05, Fig. 1B).
The rates showing the normal development were 98.4±0.5% in the control group and 97.8±0.1% in the experimental group at the DMSO concentration of 5.0%, showing no significant difference (P>0.05). Though the normally developed fish rates in the experiment group at the DMSO concentrations of 7.5% and 10.0% were 97.2±0.6%, 95.9±0.2% showing lower rates than the control group (P<0.05, Fig. 1C).
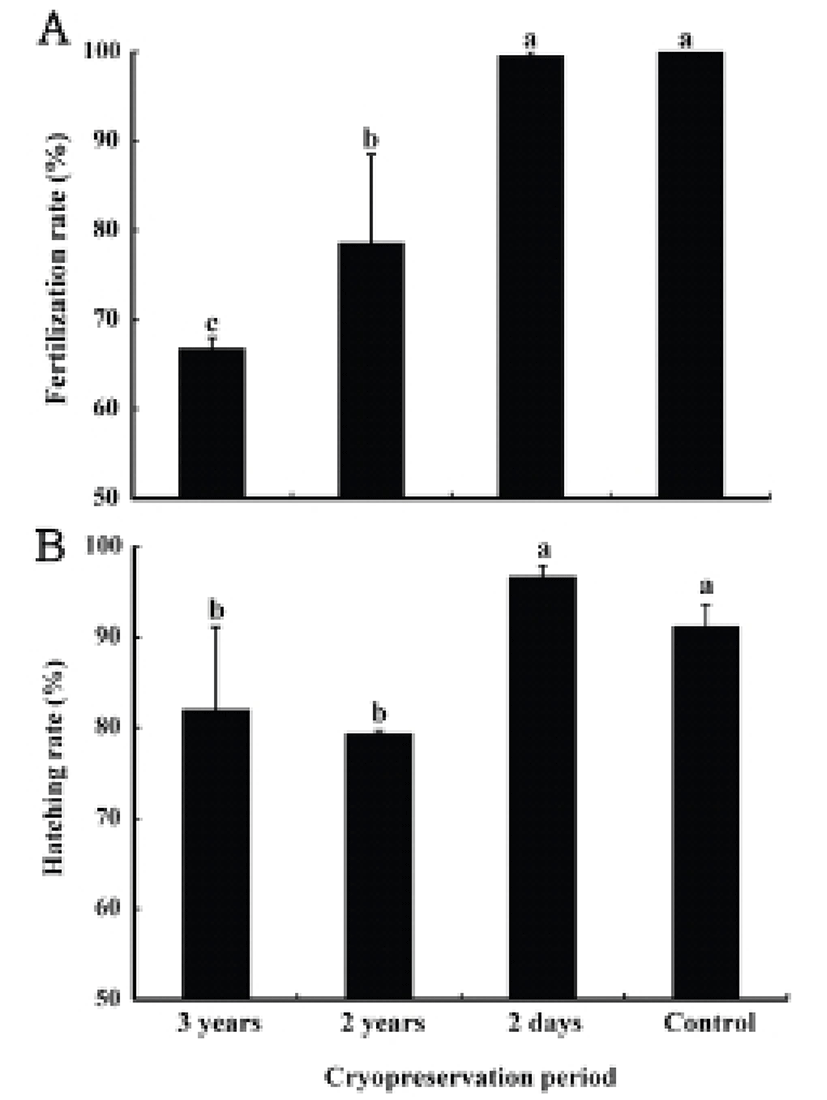
The fertilization rates at the cryopreserved periods of 2 days, 2 years, and 3 years were 99.6±0.6%, 78.5±14.8%, and 66.8±1.8%, respectively, showing the highest rate in the 2 days group (P<0.05). The control group, with fresh semen, showed exactly the same result as the 2 days experimental group (P>0.05, Fig. 2A). The hatching rates in the experimental groups at different cryopreserved periods of 2 years and 3 years were 79.3±0.6% and 82.0± 12.9% respectively showing no statistically significant difference (P>0.05). The 2 days experimental groups and the control group also showed similar results showing 96.6±1.8%, 91.1±3.6%, respectively (P>0.05). However, the control group and the 2 days sample group represented higher rates than other sample groups (P<0.05, Fig. 2B).
DISCUSSION
Semen cryopreservation is a successful procedure to preserve sperm of fish that have difficulties to secure male fish because they are protogynous hermaphrodite. For the issues that make it difficult to get sperm such as: being protogynous hermaphrodites, inconsistent sexual maturation age and size, and gender imbalance, the semen cryopreservation is a successful method to solve the issues. Various diluents and cryoprotectants have been used to cryopreserve the sperms (Bolla et al., 1987; Gwo et al., 1991; Babiak et al., 1995).
Osmotic concentration of the diluents, for the sperm cryopreservation, should be similar with the ones of the sperm, so the diluents can inhibit the sperm motility. Also the components are to be similar with the ion composition (Jamieson, 1991). The combined diluents of sodium chloride, glucose, and sucrose were used for the sperm cryopreservation of the Atlantic croaker Micropogonias undulates. Mounib's solution and marine fish Ringer's solution were effective for the sperm cryopreservation of the Atlantic halibut Hippoglossus hippoglossus (Bolla et al., 1987; Gwo et al., 1991). The black grouper E. malabaricus showed the highest sperm motility in the combination of 150 mM of NaCl and Ringer's solution for marine fish (Gwo, 1993).
5% glucose and Ringer's solution for marine fish were mixed as the diluents for the sperm cryopreservation of Epinephelinae fish, which represented no difference in the fertilization rates and hatching rates from the fresh semen group. This results in the osmotic concentration and pH in Ringer's solution, as 350 mOsm/kg and pH 7.39, were similar with the ones in the plasma of sevenband grouper E. septemfasciatus plasma as 395 mOsm/kg, pH 7.30 (Song, 2004).
Glycerol, DMSO, and methanol are the mainly used as cryoprotectants (Gwo & Arnold, 1992; Zhang et al., 2003). DMSO generally gives successful results for most marine fish such as olive flounder Paralichthys olivaceus and turbot Scophthalmus maximus because of the fast penetration into spermatozoa, its interaction with the sperm membrane, and not affecting on sperm motility during the freezing or thawing (Dreanno et al., 1997; Zhang et al., 2003). For the black grouper E. malabaricus, it showed more sperm motility when 20.0% of DMSO was used than when glycerol or methanol was used (Gwo, 1993). As for the sperm cryopreservation of the sevenband grouper E. septemfasciatus, 5.0% of DMSO represented better fertilization ability than the group with a yolk buffer (Song, 2004).
As for the fertilization rates and hatching rates of frozen-thawed sperms in the longtooth grouper, it showed no significant difference based on DMSO concentrations (5.0%, 7.5%, and 10.0%). The rates to have normally developed larvae, in the group of artificially fertilized eggs from the frozen-thawed semen, were from 97.8 to 95.9%, which is lower than the control group with fresh semen, as it is 98.4%. The abnormality occurs when artificially fertilized eggs from the thawed sperms had been genetically changed during the freezing and thawing processes and not developed normally (Julia et al., 2004; Miskolczi et al., 2005). The grass carp Ctenopharyngodon idellus had abnormality rates depending on the DMSO concentration, showing that the more concentrated cryoprotectans are used, the higher deformation rates. This assumes that there was a genetic change caused by cryopreservation (Dang et al., 2006).
The fertilizing ability in the 3 year frozen group had the fertilization rate of 66.8% and the hatching rate of 82.0%, representing lower fertilization rates and hatching rates than the control group with fresh semen. Flatfish (Zhang et al., 2003) and grass carp (Dang et al., 2006) showed the fertilization rates of 40 to 50% when they were artificially fertilized with fresh semen but the hatching rates were 90%. In addition, African clariid catfish (Oteme et al., 1996) had a 78.9% hatching rate from the 8 month cryopreserved sperm. However, this present study demonstrates that the two year cryopreserved sperm group and the three year cryopreserved sperm group has a higher fertilization rates as in 79.3% and 82.0%, which indicates successful usage. Thus, it suggests that 10% or less of DMSO and Ringer’s solution for marine fish are optimal diluents for the long term semen method. Also, the longer the cryopreservation period of sperm, fertilizing ability is decline and increase abnormality rate. It is more efficient to set an appropriate period for the cryopreservation to produce better quality eggs.

