INTRODUCTION
Puberty is the period which their sexual organs develop and become capable of having reproductive ability for the first time. Pubertal development in fish is mainly regulated by the system, so called brain-pituitary-gonad (BPG) axis (Schulz & Goos, 1999; Okuzawa, 2002; Patino & Sullivan, 2002).
Gonadotropin releasing hormone (GnRH) is a primary factor in the BPG axis, further two and more generally three forms are present within a vertebrate and between species these forms can vary in their location of expression within the brain (Weltzien et al., 2004; Whitlock et al., 2006). Several types of GnRHs have also been discovered in fish, especially a type of GnRH produced from preoptic area is known that regulates synthesis and release of gonadotropin (GTH) (Oka, 1997; Goos et al., 1998; Okuzawa & Kobayashi, 1999). Moreover, the implication of GnRH expressions in pubertal development has been suggested with some fish (Holland et al., 2001; Okuzawa et al., 2003). In the pituitary, GnRH neurons innervate gonadotrophs in order to release two types β-subunit of GTHs, i.e., follicle stimulating hormone-β (FSHβ) and luteinizing hormone-β (LHβ). The relative importance between both subunits on pubertal development of fish was often compared in many studies; nevertheless, those critical roles and/or significance are unclear (Okuzawa, 2002). The released GTHs regulate gonadal activities such as gametogenesis and steroidogenesis (Nagahama, 2000). In the gonads, subsequent steroid hormones, representatively estradiol-17β (E2) in female, are produced by the actions of GTHs and other stimulating hormones, and the increasing of their activity becomes a reason for gonadal development. Overall, the possible roles of such factors in fish reproduction are generally well-known at the present day, whereas the mechanisms of systematic circulations of these factors are still not fully understood. Therefore, observation of level changes in endocrine regulation factors underlying BPG axis is very important tools to understand pubertal development mechanisms.
Initiation of puberty in fish is correlated with the attainment of a threshold bodyweight (Huang et al., 1998). A number of studies have been suggested that age and body growth are deeply concerned with pubertal development in fish, and it varies from species to species to reach the puberty (Le Bail, 1988; Le Gac et al., 1993; Amano et al., 1997a; Taranger et al., 2010).
The longtooth grouper, Epinephelus bruneus is one of the very commercially valuable species in Korea (Lee et al., 2008; Sao et al., 2012). However, it is a protogynous hermaphrodite, and takes a long time to reach the puberty. These make it difficult to manage the broodstock and to understand the sexual characteristics of this species. Thus, classification by the bodyweight seemed suitable to understand the characteristics of pubertal development progress, further application to controlling of puberty. To achieve that, we investigated the level changes of GnRH gene (sbGnRH) in the brain, gonadotropin subunit genes (FSHβ and LHβ) in the pituitary and serum concentration levels of steroid hormone (E2), furthermore the gonadal development was observed during the process of pubertal development in female E. bruneus.
MATERIALS AND METHODS
Experimental fish were classified into three groups, approximately 1 kg group (1.1 ± 0.1 kg), 2 kg group (2.3 ± 0.2 kg), and 3 kg (3.3 ± 0.2 kg) group, according to the bodyweight. Specimens were individually marked via insertion of micro-tag (Trovan Electronic Identification System, USA) into the dorsal skin, and were maintained indoor rearing tank (6.5 × 11 × 3 m) under flow-through system. Two female fish in each group were collected on non-breeding season (April) and breeding season (June). Fish were anesthetized with 2-phenoxyethanol (Sigma Co., USA; 20 ppm), then weighed and bled from caudal vein. Each target tissues were collected by suitable methods as described below.
The gonads were removed from the abdominal cavity and fixed in Bouin’s solution for histological observation. Tissues were dehydrated in a graded series of ethanol, embedded in paraffin and then cut into 7 μm cross sections. Slides were stained with Hansen’s hematoxylin and 0.5% eosin then observed using a light microscope (HBO 50, Carl Zeiss).
The brain and pituitary were frozen in liquid nitrogen and then stored –80°C until extraction of total RNA. Total RNAs were isolated using RNAiso reagent (Takara, Co., Japan) following the manufacturer’s directions. Moreover, added Ethachinmate to aqua-phase on precipitation for carrier. Both quantitative and qualitative analyses of total RNAs were performed by Optizen 2120 UV spectrometer (Mecasys Co., Korea) and Nanodrop 1000 (Thermo Scientific, Waltham, MA, USA). Reverse transcript reactions contained 5 μg total RNA in Diethyl-pyrocarbonate (DEPC) distilled water. Total RNAs were heated at 65°C for 10 min, then immediately chill on ice for 2 minute. The reactions were added with 0.5 μg Oligo-dT15-mer (Promega, USA) primer in Ready-To-Go-You-Prime First-Strand Beads (GE Healthcare, UK) kit. The reactions were incubated at 37°C for 60 min for cDNA synthesis. Primers for quantitative real-time PCR analysis were based on cDNA sequences for E. bruneus sbGnRH, FSHβ and LHβ (GeneBank accession number: FJ380047, EF583919, EF583920). The primer sequences and product sizes are shown Table 1. Twenty-five μl of real time RT-PCR reaction mixtures contained 12.5 μl of IQ supermix (Bio-Rad, USA), 5 μl of cDNA, 1 μl (500 nM) of each primer and probe. The PCR amplification was carried out on an Chromo 4™ Four-Color Real-Time System (Bio-rad, USA) as follows: denaturation at 50°C for 2 min and then 95°C for 3 min, 45 cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 1 min. Each sample was analyzed in duplicate.
Serum E2 levels were measured by radioimmunoassay followed by Aida et al. (1984). Minimum detectable limit was 12.73 pg/ml. Intra-assay and inter-assay coefficients of variance were 6.02% (n=3) and 9.20% (n=6) on assays.
All data were represented as means ± standard error and subjected to one-way ANOVA analysis of variance followed Duncan's multiple range test (Duncan, 1955). Differences were considered at P<0.05.
RESULTS
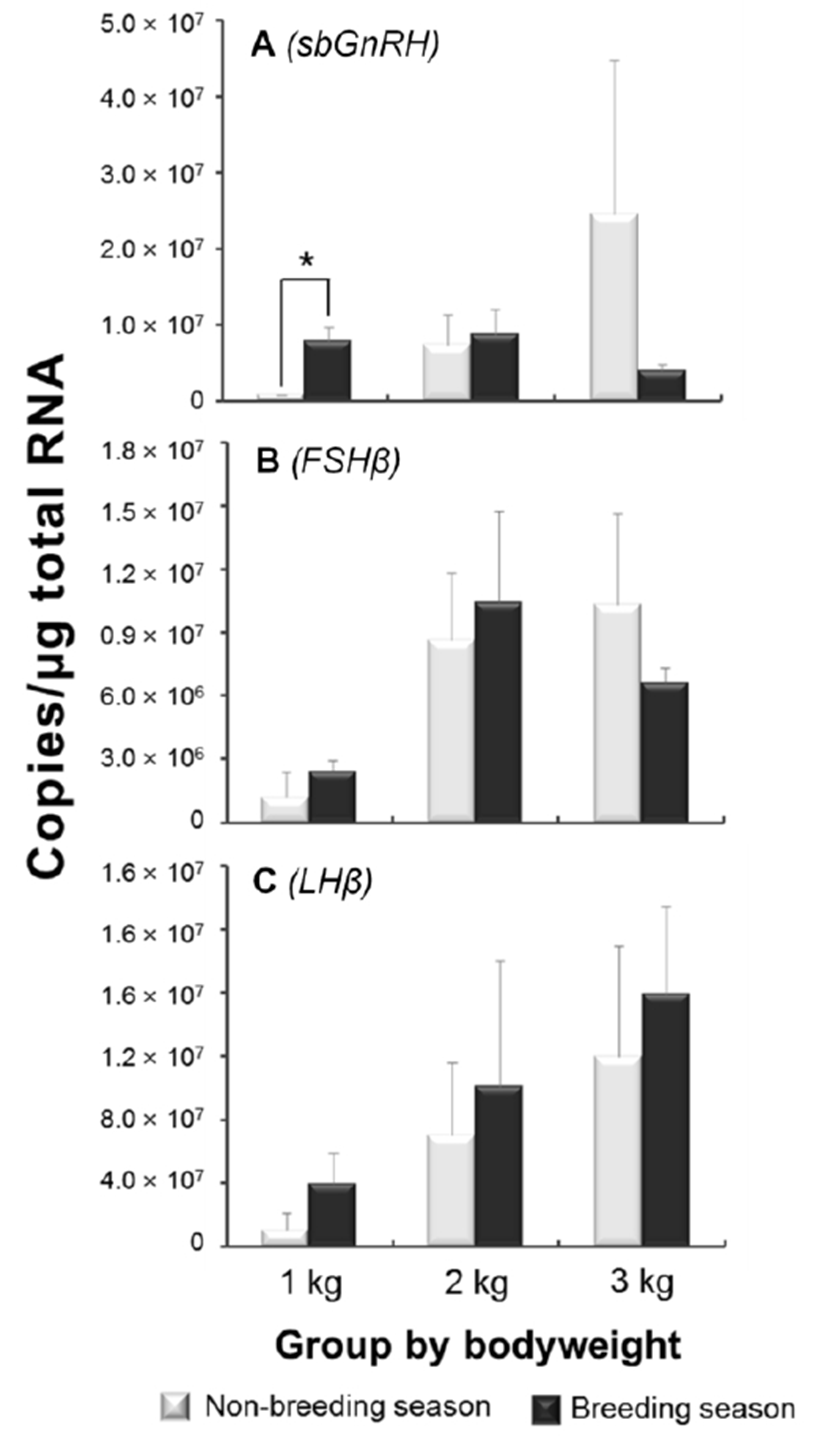
In the quantification of sbGnRH and GTHs mRNA expression levels, significant differences between the seasons were not observed in all groups except sbGnRH mRNA levels in 1 kg group (Fig. 1). The expression levels of sbGnRH in non-breeding season showed increasing trends in accordance with bodyweight gaining; especially, large variation was observed in 3 kg group. On the contrary, there were no remarkable level changes in breeding season (Fig. 1A). The expression levels of both GTH subunits also showed increasing trends with bodyweight gaining. In both seasons, the FSHβ mRNA levels rapidly increased from 2 kg groups although the lower level than non-breeding season was observed in 3 kg group (Fig. 1B). The LHβ mRNA levels showed clear increasing trends by the bodyweights and the levels of all groups in breeding season were higher than non-breeding season (Fig. 1C).
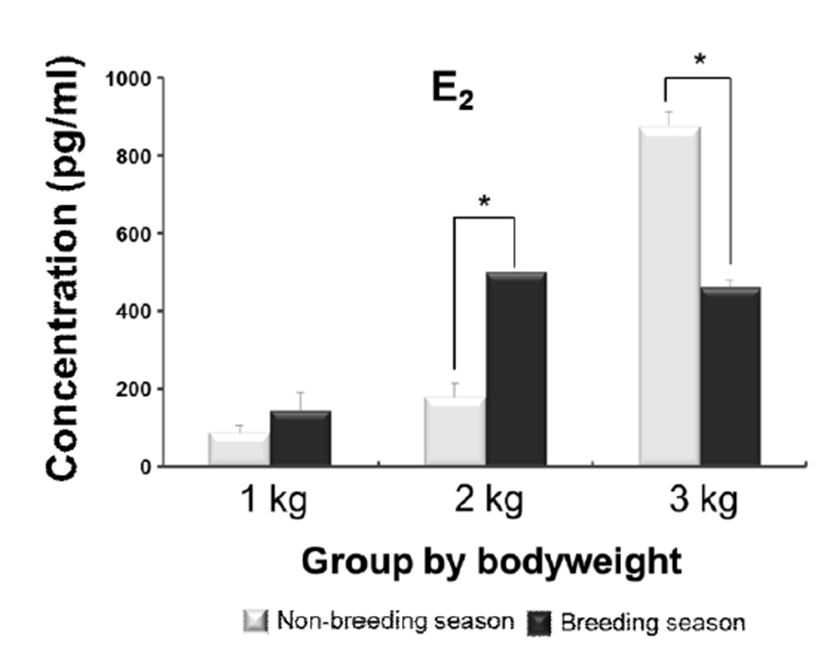
Likewise the serum concentration of E2 showed increasing levels coinciding with bodyweight gaining. The level of 3 kg group was dramatically increased in the non-breeding season, as compared to the moderate level seen in breeding season. The significant differences were also observed in 2 kg and 3 kg groups between the seasons; the level of 2 kg group in breeding season was significantly higher than non-breeding season, however the trend was reversed in 3 kg group that the level in non-breeding season was higher than breeding season (Fig. 2).
In the non-breeding season, the ovaries of both 1 kg and 2 kg groups were mostly composed under peri-nucleous stage oocytes. The 1 kg group contained peri-nucleous oocytes having approximately 80 μm of diameter (Fig. 3A), and 2 kg group showed similar gonadal development phase while the oocytes were homogenous sizes than 1 kg (Fig. 3B). In the ovaries of 3 kg group, more developed oil-droplet stage oocytes with approximate 150 μm of diameter were observed (Fig. 3C).
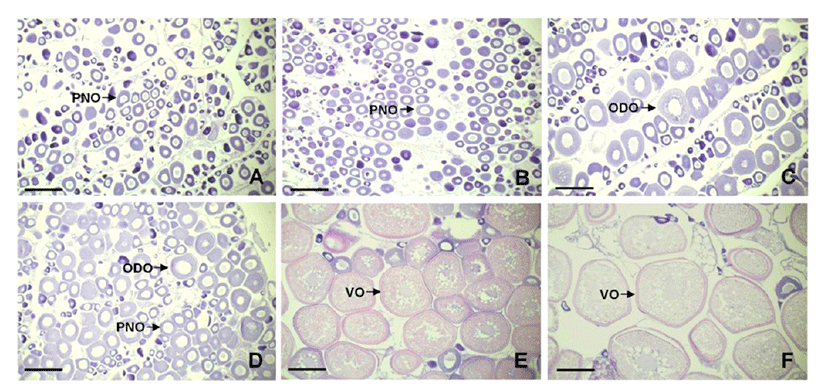
In the breeding season, 1 kg group still contained perinucleous oocytes and partly oil-droplet oocytes (Fig. 3D). On the contrary, the ovary of 2 kg group was matured than 1 kg group showing vitellogenic oocytes accumulated abundant yolk granules within ooplasm (Fig. 3E). Among the groups, the 3 kg group showed most matured ovary that composed greater part of vitellogenic oocytes of approximately 450 μm diameter (Fig. 3F).
DISCUSSION
Puberty in fish is regulated by some key factors, such as GnRHs, GTHs and steroid hormones, involving activation of BPG axis. Nevertheless, the mechanisms underlying the initiation of puberty are still unclear. Therefore, in this study, we have attempted to characterize the pubertal development phases of female E. bruneus, based on the expression patterns of such endocrine regulation factors in accordance with bodyweight gaining.
Fish have several types of GnRHs (Lethimonier et al., 2004). It has been reported that three forms of GnRH are representative in perciformes, i.e., red seabream type of GnRH (sbGnRH), salmon type of GnRH (sGnRH) and chicken type of GnRH (cGnRH-II) (Amano et al., 1997b). Especially, sbGnRH are known as major type for reproduction in perciformes species (Senthilkumaran et al., 1999; Pham et al., 2006). Thus, seabream type of GnRH was previously cloned from the brain of E. bruneus (data not shown) and the mRNA expression levels were investigated in this study. The expression levels of sbGnRH mRNA showed increasing trend during the progress of pubertal development in non-breeding season. Such patterns were also observed in other species, such as Oncorhynchus masou, Pagrus major, Dicentrarchus labrax and Morone saxatilis (Amano et al., 1997a; Rodríguez et al., 2000; Holland et al., 2001; Okuzawa et al., 2003) during pubertal development, indicating that activation of sbGnRH expressions is possible to be trigger of onset and development of puberty. In the breeding season, however, remarkable levels increasing of sbGnRH expression was not observed, and the level was lower than non-breeding season in 3 kg of group. This result suggests that peak of sbGnRH mRNA expression in breeding season was already completed, and consequently decreased at low levels in around 3 kg of female E. bruneus. Evidently, the expressions of sbGnRH were decreased when they finished pubertal development in many perciformes (Senthilkumaran et al., 1999; Collins et al., 2001; Okuzawa et al., 2003). Therefore, the peak of sbGnRH mRNA expressions in non-breeding season of 3 kg group seemed a sign the entry to puberty in female E. bruneus.
The teleosts GTHs are assumed as key factors for regulating gonadal maturation (Weltzien et al., 2004). In the present study, both FSHβ and LHβ mRNA expression levels exhibited increasing trends through the progress of pubertal development along with bodyweight gaining. In general, FSH is supposed that has more important influence than LH on pre-pubertal and early pubertal development in teleosts. However, those crucial roles in onset of puberty still remained as controversial issue due to the levels varying degree with species. The superior levels of FSHβ compared with LHβ during early puberty have been reported in O. masou, Oncorhynchus mykiss and M. saxatilis (Amano et al., 1992; Gomez et al., 1999; Hassin et al., 1998; 2000). On the contrary, relative importance of LHβ gene expressions on progress of puberty was also reported in D. labrax and P. major (Gen et al., 2000; Rodríguez et al., 2000). In this study, the LHβ expressions sustained increasing trend, further the absolute copy numbers were higher than FSHβ during pubertal progress. These phenomena mean the roles of LHβ on puberty may be important at least in E. bruneus, although the wide variety with fish species remained to be verified yet.
One of the potential factors effecting on the initiation and development of puberty in several fish species appeared to be gonadal sex steroids (Okuzawa, 2002); an increase in the plasma E2 levels was coincided with sexual maturation (Campbell et al., 2006). Such increase in plasma E2 associating with the initiation and progress of oocyte development phase has also been reported in Carassius auratus and Danio rerio (Khoo, 1979; Kwok et al., 2005). Thus, the increased levels of E2 in this study seem to support the promoting effects of E2 on the pubertal development of female E. bruneus. Moreover, the decrease in levels of 3 kg on breeding season is supposed the positive influence by feedback of steroid hormones; because this group seemed already enter the puberty phase.
The level changes of endocrine regulation factors which described above are directly reflected on the oocytes developments. In the histological observations, we could confirm clear differences with oocyte developmental stages by the bodyweight gaining as well as pubertal development progress. In the non-breeding season, remarkable differences could not observed among the groups, while the definite differences appeared with 2 kg and 3 kg groups in breeding season; notably, the oocytes contained 3 kg group were most matured. These are supposed immediate evidences that consequent on the expressions of endocrine factors. Because, it is well-known that the combinations of increases in FSHβ and E2 levels stimulate oocyte development in several fish species (Campbell et al., 2006). Therefore, high expressions of FSHβ mRNA and serum E2 levels of 3 kg groups in non-breeding season are involved in accumulation of lipids and vitellogenin into oocytes, resulting reach the oocyte maturation in breeding season.
Taken together, 1 kg of female E. bruneus was classified pre-pubertal stage, charactering any endocrine regulation factors still not fully expressed and the ovary was composed under peri-nucleous oocytes. On the other hand, 2 kg and 3 kg groups can be classified early-pubertal stage based on the sufficient levels in endocrine regulation factors and matured oocytes. However, we supposed that 3 kg group is already possible to spawn (puberty phase), because that having over 450 μm oocytes in gonads which is essential requirement for inducing of final oocyte maturation in reared groupers (Marino et al., 2003; Lee et al., 2008). In general, rearing condition is not suitable to complete gametogenesis in groupers owing to insufficient of environmental cues. Thus, if suitable solutions be provided, e.g. hormonal treatments, the 3 kg group will achieve the final oocyte maturation.
In summarized, our results indicate that pubertal development progress in female E. bruneus is able to classify following three phases; pre-puberty (1 kg), early-puberty (2 kg) and puberty (3 kg). Moreover, this research useful in future works to control of puberty that is necessary for the successful management of broodstock in E. bruneus.

