INTRODUCTION
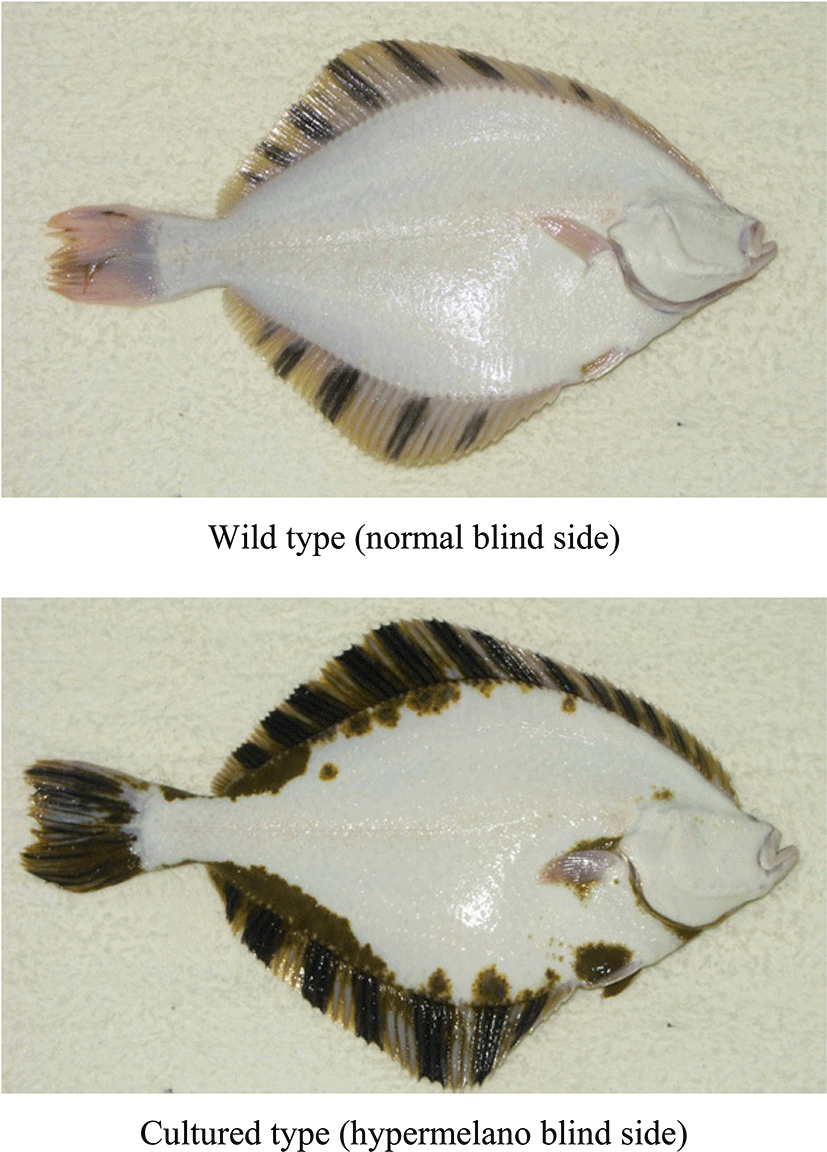
The starry flounder, Platichthys stellatus, is a marine demersal heterosomatic fish (Pleuronectiformes) with both eyes located on the same side of the body (Byun et al., 2007; Ivankova & Ivankov, 2006; Kang et al., 2012). The fish is bilaterally symmetrical after hatching, but becomes laterally asymmetrical after metamorphosis. During metamorphosis, the right (or left) eye migrates to the left (or right) side and occupies a position beside the left eye. The skin is asymmetrically pigmented on the ocular side (Byun et al., 2007). From hatching to metamorphosis, this fish has symmetrical body skin in which larval-type melanophores are evenly distributed. However, at the peak of metamorphosis, small adult-type melanophores, xanthophores, and iridophores are rapidly formed on only the ocular side. After metamorphosis, the ocular-side skin shows a distinct pigmentation pattern, but the blind-side skin shows no pigment pattern (Byun et al., 2007). However, the blind-skin malpigmentation of flatfish that accompanies mass production in hatcheries is a major problem (Fig. 1). As summarized by VenizelosBenetti (1999) and BolkerHill (2000), such malpigmentation has occasionally been observed in other wild-caught flatfishes, but is extremely rare in wild populations. The strong commercial value of starry flounder has made it a promising species for aquaculture and resource enhancement in South Korea; indeed, considerable mass aquaculture production is currently underway. However, the symptom is abnormally common in hatchery flatfishes, with up to 95% of hatchery-reared populations showing black areas on the blind side (Kang et al., 2011). In fact, malpigmentation is often used to distinguish cultured from wild fish. Especially, this malpigmentation is considered an important problem with regard to resource conservation, marketing, and circulation of fishery products and the culture industry. In wild ecosystems, when hypermelanic flounders are released to enhance coastal resources, they could distort the natural inherited characteristics of starry flounder living in the wild. In addition, the malpigmentation is economically important in the fisheries industry. As malpigmented cultured flounders are sold at a lower price than ordinary flounders, blind-side malpigmentation is a common concern in culturing the fish due to customer preference.
Although many researchers have suggested that the cause of blind-side malpigmentation is the background color of the tank (Amiya et al., 2005; Stickney & White, 1975), the brightness of light in the culture environment (Denson & Smith, 1997; Iwata & Kikuchi, 1998), diet (Hamre et al., 2005; Takeuchi et al., 1995), and bottom substratum and type of tank (Iwata & Kikuchi, 1998; Kang & Kim, 2012), the precise reason has not been clarified. In addition, we do not have sufficient knowledge regarding the mechanism underlying the appearance of malpigmentation. Therefore, methods of preventing or diminishing blind-side hypermelanosis in starry flounder artificially cultured at high densities are required. We are attempting to develop technical solutions based on an overall understanding of developmental malpigmentation on the blind side in flounder, but lack basic knowledge of the symptoms as they appear during mass production aquaculture. Before techniques to control undesirable pigmentation in fish raised in large-scale facilities can be developed, it is first necessary to determine when blind side hypermelanosis in starry flounder manifests during mass production aqua-culture, elucidate the morphological and quantitative processes involved in this malpigmentation, and deduce its causes and mechanisms. To understanding the developmental properties of blind-side hypermelanosis, we analyzed the asymmetrical differentiation process of chromatophores during early development of starry flounder, and examined subsequent morphological pattern changes after metamorphosis.
MATERIALS AND METHODS
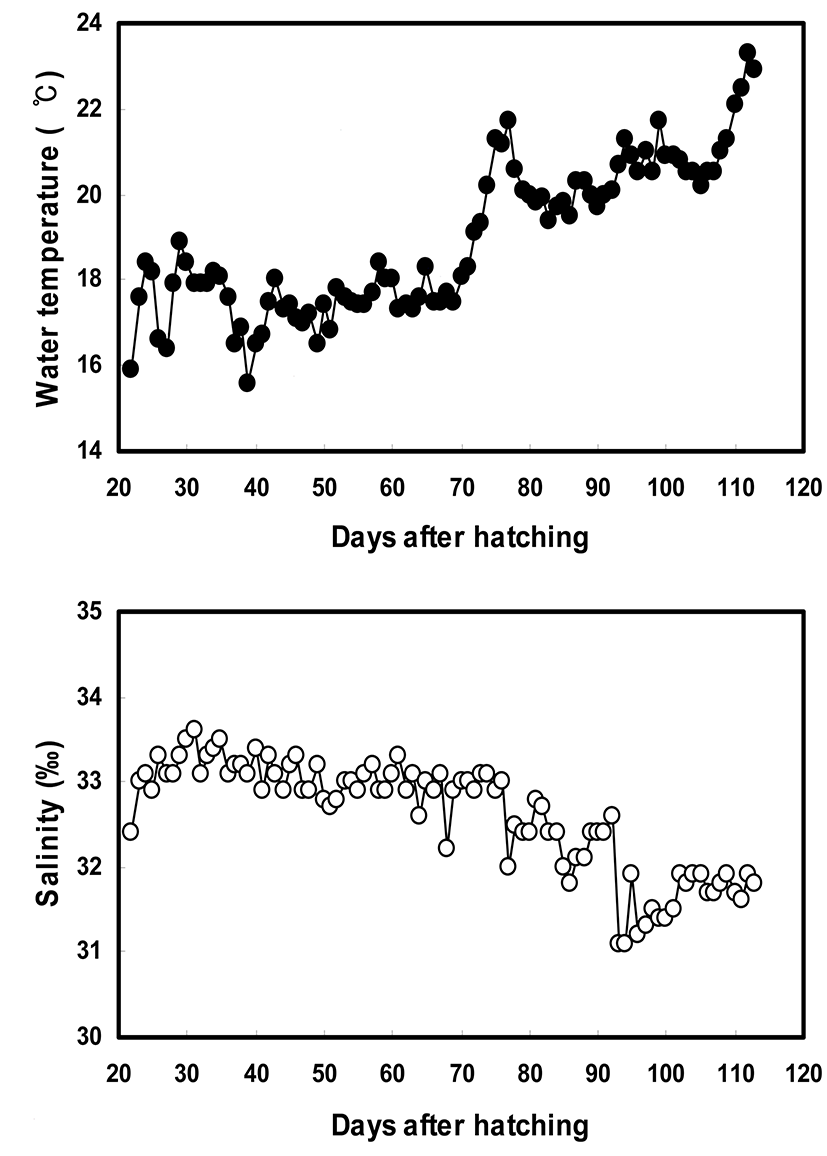
In this study, we examined the pigmentation expression pattern on the blind side of starry flounder using the larvae from 22 days after hatching [DAH] (total length [TL]= 10.04±0.21 mm, body weight [BW]=8.8±0.5 mg]. Observations were made during the 88-day period from 22 to 110 DAH. Larvae were reared under natural photoperiod in tanks with water at a temperature of 15.6~23.3°C and salinity of 31.1~33.6 psu (Fig. 2). As food, the fry at 22~48 DAH were supplied rotifers and Artemia nauplii, while Artemia nauplii and commercial pellets were provided at 49~100 DAH.
Thirty flounders (n=30 animals per sample point) were collected from each tank using a net at an interval of 7 or 10 days. The sampled fish were fixed in 10% neutralized formalin immediately after sampling. The fish were not fed for 24 h prior to sampling, and were anesthetized with 2-phenoxyethanol (1/1,000 dilution, 0.3~0.4 mg/L) and rinsed with distilled water to remove salt. The TL and BW of each animal were measured at an interval of 7 or 10 days. The total body surface area covering the bottom of the aquarium was also calculated and converted into PCA (percentage of covering area, %). The blind sides of the sampled flounders were photographed with a digital camera (Dimage A2; Konica Minolta, Tokyo, Japan). The ratio of pigmented area of blind side (pigmented area of blind side/total area of blind side×100%) was analyzed with a microimaging analysis system (Qwin; Leica, Wetzlar, Germany) using the photographs. Individuals with a pigmented area of more than 1% on the blind side were considered ambicolored. The numbers of ambicolored individuals in each group were counted, and the ambicolored fish ratio (no. ambicolored fish/no. total fish× 100%) was calculated at 7- or 10-day intervals.
Statistical analyses were performed to determine the significance of differences in mean values between the groups in each experiment. The pigmented area ratio and the ambicolored fish ratio were compared using the means of the 30 pooled individuals (n=30). Student’s t test or one-way ANOVA for parametric data (n>30), and the Kruskal-Wallis test or Mann–Whitney U-test for non-parametric data (n<30) were applied using the statistics software SPSS 7.0 (Korean version 7.50; SPSS Inc., Chicago, IL) with a 95% confidence level to compare differences in the data.
RESULTS
At 24 DAH, before the body had transformed, the right (or left) eye of the flounder began to migrate toward the left (or right) side; in most fish, the migrated eye moved as far as the dorsal trunk ridge. Notochord flexion began, and the body started to flatten laterally. The body remained transparent on both sides. Many large (larval-type) melanophores, xanthophores, and iridophores were evenly distributed in the skin on both sides (Fig. 4). The larvae were 10.0±0.2 mm in length and weighed 8.8±0.5 mg at this stage (Fig. 3).
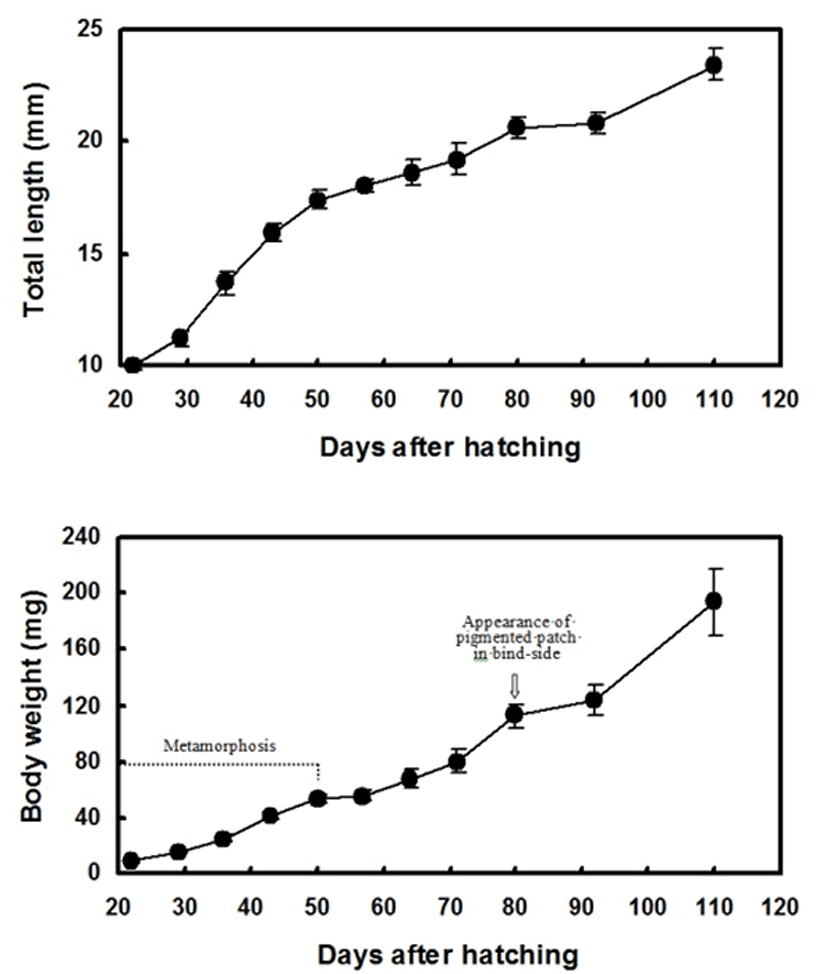

The peak of metamorphosis, when migration of the eye was complete, occurred at 29 DAH. The lateral line was faintly distinguishable, and the fin ray and pectoral fins were clearly visible. Larval-type chromatophores were still observed on both sides. The ocular-side skin was pigmented by chromatophores, xanthophores, and iridophores, but pigment cells were diminished on the blind-side skin, which was still translucent (Fig. 4). At this stage, the larvae were 11.2±0.3 mm in length and weighed 16.0±1.1 mg (Fig. 3).
At 36 DAH, the opposite side eye and intestines were visible through the transparent blind side. A pair of pectoral fins could be seen, and the number of larval chromatophores was decreased. The primitive pigmented spots composed of adult-type melanophores were slightly visible around the anus on the blind side by microscopic observation. However, the pigmented spot composed of adult-type melanophores on the blind side was not yet visible to the naked eye. Some of the flounders completed metamorphosis, and the first novel feature appeared (Fig. 4). At this stage, the fish were 13.7±0.5 mm in length and weighed 25.0±2.4 mg (Fig. 3). At 43 DAH, the flounders were still undergoing metamorphosis, and some fish had settled on the bottom of the tank. The body became weakly transparent (Fig. 4). At this stage, the fish were 15.9±0.4 mm in length and weighed 40.8±2.7 mg (Fig. 3).
At 50 DAH, the lateral line was clearly distinguishable. Most of the flounders were completely at the juvenile stage of development. The number of larval-type melanophores on the blind side decreased. Adult-type melanophores were more numerous around the trunk between the pelvic fin and anus than in the previous stage. The first pigmented large spot (patch) was first observed by the naked eye around the trunk between the pelvic fin and anus in some of the flounders. However, the pigmented patch composed of three adult-type chromatophores on the blind side was not present elsewhere (Fig. 4). At this stage, the juveniles were 17.4±0.4 mm in length and weighed 53.6±3.5 mg (Fig. 3).
At 57 and 64 DAH, the body was weakly transparent and the blind side became white in color. The pigmented patch could be seen by the naked eye around the trunk between the pelvic fin and anus in most of the flounders. The blind-side epidermis was a distinctive white color and was markedly less transparent, with the intestines no longer visible to the naked eye. Larval-type melanophores were still present in the blind-side dermis (Fig. 4). At this stage, the juveniles were 18.0~18.6 mm in length and weighed 55.4~67.9 mg (Fig. 3).
At 80 DAH, slight pigmentation of adult type was observed for the first time during development around the caudal peduncle on the blind side. In some fish, additional pigmented patches were first observed near the rear trunk beside the dorsal fins on the blind side. The pelvic fin was obviously pigmented. However, larval-type melanophores remained evenly distributed on the blind side (Fig. 4). At this stage, the juveniles were 20.6±0.5 mm in length and weighed 112.5±8.8 mg (Fig. 3).
From 92~110 DAH, the overall body shape resembled that of fish from the previous developmental stage, but a new feature appeared. Black pigmented patches composed of three adult-type chromatophores were more evident and were widespread around the trunk next to the rear dorsal fins on the blind side. Black spots composed of adult-type melanophores appeared around the pectoral fin and front trunk beside the dorsal fins, and on the eye vestiges on the blind side (Fig. 4). The spotted area on the blind side grew larger and darker. The mean TL increased from 20.8±0.5 to 23.40±0.7 mm, while the BW increased from 123.5± 10.8 to 193.6±23.3 mg (Fig. 3).
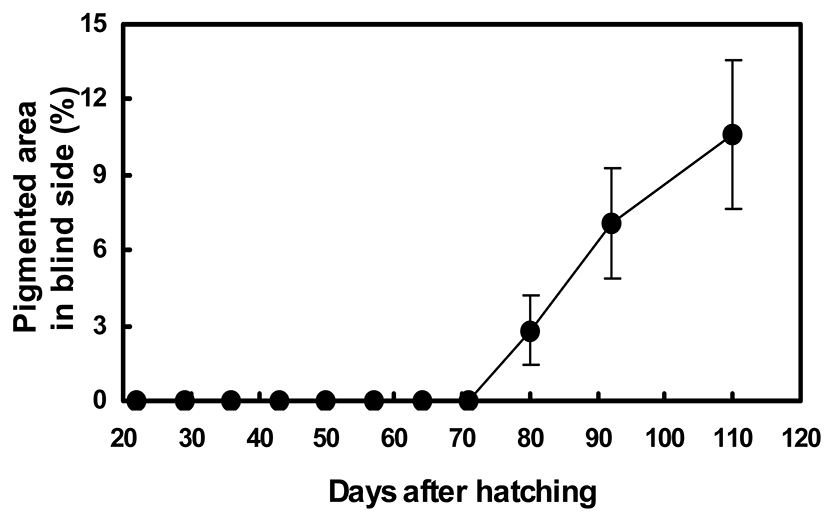
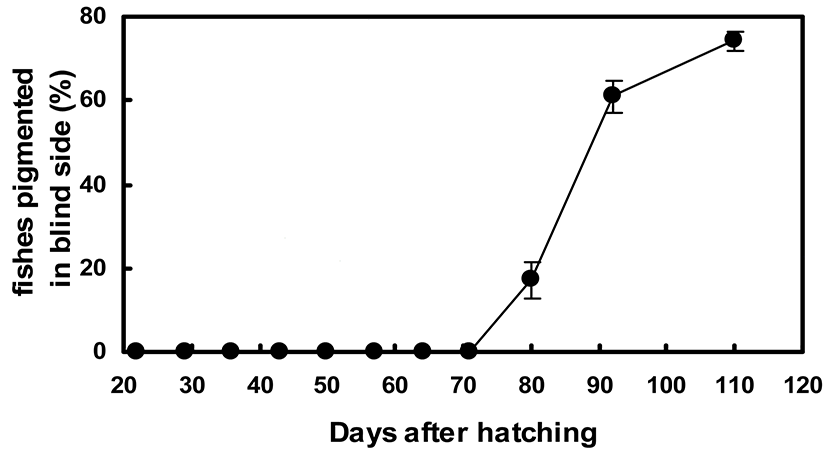
Figures 5 and 6 show the quantitative changes in pigmentation pattern on the blind side from 24 to 110 DAH. From 20 to 70 DAH, no pigmented black spots composed of adult-type melanophores were found on the blind side. The primitive spot could first be seen by the naked eye on the rear trunk beside the dorsal fins at 80 DAH (Figs. 4 and 5). At 80 DAH, the percentage of staining area was extremely low (2.8%±1.4%), but the percentage of malpigmented fish was significantly high (17.1%±4.2%) (Figs. 5 and 6). Up to 110 DAH, the staining area ratio increased markedly with growth to 10.6%±3.0%. The percentage of fish showing blind-side pigmentation increased markedly to 74.2%±2.2% at 110 DAH (Figs. 5 and 6).
DISCUSSION
Fish show two types of skin color change. The first is physiological color change (e.g., camouflage) in which fish match their color and skin pattern to those of the surrounding environment, while the second is irreversible skin color change due to the differentiation and development of chromatophores with growth. The former is a physiological color change, involving reversible movement of pigment in chromatophores to rapidly and transiently match skin pattern and colors to the surroundings (Ellis et al., 1997; Ramachandran et al., 1996) while the latter is a morphological change involving irreversible differentiation and development of chromatophores causing a quantitative increase in pigment. The hypermelanosis of blind-side skin of starry flounder observed in the present study is an example of the latter type of color change.
The ordinary starry flounder has numerous chromatoblasts just before metamorphosis. The chromatoblasts either differentiate into pigment cells or remain as stem cells, and are evenly distributed on the left and right epidermal sides. As the eye gradually migrates to one side during metamorphosis, the stem cells in the skin on the ocular side differentiate into three adult-type pigment cells (melanophores, iridophores, and xanthophores). However, the chromatoblasts on the non-ocular side gradually shrink and collapse during metamorphosis, and some later differentiate into iridophores. The result of these processes is that the starry flounder is asymmetrically colored after metamorphosis. The ocular side is variously colored. The non-ocular (blind) side is white. These observations confirmed earlier findings in other flatfishes reported by Minami (1982), Seikai (1992), and MatsumotoSeikai (1992). However, in our study, hypermelanic flounders showed abnormal differentiation of the chromatophores in a limited area of blind-side skin after metamorphosis, and most flounders exhibited malpigmentation on the blind side. Similar to the ocularside skin, a few pigment patches comprised of small adult-type chromatophores were observed near the dorsal fins on the blind side, indicating that these patches are not made by transient movement of pigment in chromatophores, but were formed irreversibly by differentiation of chromatophores. Such aberrant morphology has been demonstrated previously in many other Pleuronectiformes: olive flounder (Haga et al., 2005; Tomiyama et al., 2008); stone flounder, Kareius bicoloratus (Matsumoto & Seikai, 1992); halibut, Hippoglossus hippoglossus (Ottesen & Strand, 1996); barfin flounder, Verasper moseri (Yamanome et al., 2005); and yellowtail flounder, Limanda ferruginea (Purchase et al., 2002).
In addition, our quantitative investigation indicated that the abnormal pigmentation on the blind side showed consistent and irreversible developmental patterns. The proliferation of pigment cells in the blind-side skin was accelerated over time, and the size and number of pigmented patches increased with growth. The first pigment patch of the blind side visible to the naked eye appeared at 80 DAH. At this time, two to three pigmented patches were observed on the blind side. The average pigmented area ratio was 2~3%, and the ratio of hypermelanic fish in the community reached ~17%. Up to 110 DAH, the staining area ratio increased markedly with growth to 10.6±3.0%. The percentage of fish showing blind-side pigmentation increased markedly to 74.2±2.2%.
Similar to other heterosomatic fishes (Amiya et al., 2005; Denson & Smith, 1997; Diaz De Astarloa, 1995; Matsumoto & Seikai, 1992), the blind-side malpigmentation of the starry flounder that appears at a very low ratio in wild populations was frequently observed in juvenile starry flounders reared in artificial facilities with a flat bottom and dark background. However, it is not yet clear why abnormal blind-side pigmentation occurs in flounders artificially cultured in high-density facilities. Several possible physical and ethological factors have been suggested to explain pigment anomalies in hatchery-reared Pleuronectiformes, including stress factors, light (Itoh et al., 2012; Iwata & Kikuchi, 1998; Matsumoto & Seikai, 1992), density (Takahashi, 1994), tank color (Yamanome et al., 2007; Yamanome et al., 2005), and bottom substratum (Estevez et al., 2001; Iwata & Kikuchi, 1998; Kang & Kim, 2012; Ottesen & Strand, 1996). As hypermelanosis on the blind side of Pleuronectiformes occurs occasionally in wild flounders (Diaz De Astarloa, 1995; 1998; Stickney & White, 1975), the symptom in flounders is likely to have a strong genetic component. Abnormal differentiation of adult-type melanophores on the blind side in flounders is evidently multifactorial, and both rearing stress and morphological evolution should be taken into consideration. Mass production facilities lack bottom shelters (e.g., mud, sand, or gravel), and flounders in these facilities are vulnerable to cannibalism. They compete for food with other flounders being reared in the same tank. These environmental stressors may select for adaptive camouflage on the blind side as well as on the ocular side. If such selection occurs and the appropriate conditions hold, chromatophores on the blind side degenerated (or atrophied) through evolution would re-differentiate.
In conclusion, primitive pigmentation on the blind side visible to the naked eye first appears in the trunk region around the dorsal fins and caudal peduncle at 80 DAH. Abnormal coloration resulting from differentiation of pigment cells on the blind side after metamorphosis extends to the entire blind side as growth proceeds. However, the molecular biochemistry from which abnormal pigmentation arises is not yet known. Future studies of the molecular endocrinological mechanism underlying the abnormal pigmentation in cultured olive flounder are required to clarify the developmental aspects of abnormal pigmentation indicated in the present study.