INTRODUCTION
The grunt, Hapalogenys nitens, which belongs to the Perciformes (Haemulidae), is found in all coastal waters of Korea, south Japan, and eastern China Sea (NFRDI, 2004). The body type of H. nitens is characterized with high body height, two clear and wide dark brown colored lines on the side of body; a similar kind, crescent sweetlips, Plectorhinchus cinctus, is distinguished by spots on the back and caudal fin (NFRDI, 2004). H. nitens grows fast and represents strong resisting power against diseases, making it as a good potential fishery species for development of novel aquaculture species in southern coastal area of Korea inclu-ding Tong-young area. In recent, the National Fisheries Research and Development Institute is currently conducting preliminary investigations for species conservation in order to study Korean indigenous species conservation as well as seed production.
Previously, there have been many studies on H. nitens,: on aspects of reproduction, including vitellogenesis (Cuiqin et al., 2006), natural spawning and egg development (Kang et al., 2004a), morphological development (Xie et al., 2004), development of seed production technology (Zhang et al., 2001a; Hong and Zhang, 2002, 2003), on aspects of ecology, including distribution and morphology (Masuda et al., 1984; Lee et al., 1997; Lim and Choi, 2009), effects of water temperature and salinity on hatching and larval survival (Lin et al., 1998), effect of salinity on activity and larval feeding rate (Zheng et al., 2004), effects of feeds on growth and survival of juveniles (Zhang et al., 2003), effects of low salinity and cold water temperature on growth and survival rates of eggs and offspring (Kang et al., 2009), on aspects of aquaculture, including growth performance in cage aquaculture (Li et al., 2007), early nutritional com-positions (Zhang et al., 2001b; Limin et al., 2006), and on aspects of genetics, including karyotypes (Ziniu et al., 1994; Chen et al., 2005), genetic diversity (Liang et al., 2003), microsatellite separation for genetic analyses (An et al., 2014) of this species.
Although, there are several studies, there are still gaps in our knowledge on reproduction and aquaculture. Little information is available on the reproductive cycle, the use of sexual reproductive hormones associated with sexual maturation and seedling production of H. nitens. Regarding the development of aquaculture technology, recently, sexual reproductive hormone control have been studied for artificial spawning and rapid growth. Thus, the use of sexual reproductive hormone control will contribute to develop aquaculture technology of this species. Hence, it is expected that information for technology development of massive seedling production of H. nitens would be clarified.
Therefore, the purpose of the present study is to describe basic information of the gonadosomatic index (GSI), repro-ductive cycle with gonadal development, and plasma sex steroid studies such as use of changes in estradiol-17β and testosterone in plasma in female and male H. nitens for aquaculture.
MATERIALS AND METHODS
A total of 30 H. nitens (total length: 38.2 ± 0.2 cm; body weight: 1,577.7 ± 31.7 g), which were maintained and cultured from August 2011 to July 2012 at the Yellow Sea Fisheries Research Institute of NFRDI, were used for the study. The culture conditions were as follow: fishes were maintained in 10 tons-concrete circular tank; natural sea water was changed 6 times per day utilizing a high pre-ssure sand filter while assorted feed for flatfish, Paralichthys olivaceus was provided twice a day. In the winter season, water temperature was heated, maintained at 11.5~26.5°C year round. And salinity was maintained with the salinity concentration of 30.1 and 33.9 psu.
When it comes to the culture management, seven stages were subdivided: spawning period (29 August 2011), after spawning (1 October 2011), before wintering (26 November 2011), wintering period (19 February 2012), after wintering (21 April 2012), culture period (27 May 2012), and before spawning (27 July 2012).
To monitor changes in hormonal levels of plasma, each one of fishes in the tank was retrieved and then plasma sample was taken from tail vein utilizing a heparin treated syringe; upon the sample was taken, the fish was immediately transferred to the tank and recovered. The plasma sample was separated by spinning at 12,000 rpm for 5 minutes using a centrifuge which was maintained at 4°C. Separated plasma sample was stored at –75°C until further analyses. Plasma steroids were extracted using the method as described previously (Aida et al., 1984); extracted steroids were then stored at –70°C for the radioimmuno-assay (RIA assay). For the quantification of steroid hormones, the RIA method, previously described in Aida et al. (1984) and Lou et al. (1984), was utilized. The estradiol-17β and testosterone were quantified in female and male fishes, respectively. Antibodies used for the quantification were obtained from Teikoku Zoki Pharm. Co.; the cross-reaction rates of these antibodies were found to be 3.2, 1.77, and 0.29% for estrone, estradiol, and testosterone, respectively.
The GSI was calculated by (gonad weight/body weight) × 100. Histological observations for both female and male gonadal developments were made; three fishes were retrieved per each culture stages, for a total of six times: before spawning (11 August 2011), after spawning (18 November 2011), wintering period (18 March 2012), after wintering (21 April 2012), culture period (27 June 2012), and shortly after spawning (16 October 2012).
For light microscopic examination of histologic pre-parations, female ovarian tissues and male testicular tissues were removed from the gonads and preserved in Bouin fixative for 24 h, and then washed running tap water for 24 h. The tissues were subjected to standard histological procedures (dehydrated in alcohol and embedded in paraffin) and sectioned at 5~8 μm using a rotary microtome. Sections were then mounted on glass slides, stained with either Hansen’s hematoxylin-0.5% eosin, and inspected under a light microscope. After histologic preparations produced by the methods mentioned earlier, prepared tissue sections were then analyzed and monitored for shapes and sizes of germ cells utilizing an optical microscope (Axioskop 2 plus; Carl Zeiss, Jena, Germany) interfaced with the image analysis system (AxioVision Rel., ver. 4.6).
To monitor ovarian and testicular developmental phases, shapes and structures of germ cells were histologically analyzed. All histological terms used for designating cellular structures were adopted as reported in the study of Grier et al. (2009) in the “Reproductive Biology and Phylogeny” published by Jamieson in 2009; recently, all terms are internationally certified and widely utilized in other studies.
All results herein were expressed as mean±S.E and statistical differences of means between groups were deter-mined using t-test and one way-ANOVA test (SPSS package, ver. 9.0) at P value of 0.05.
RESULTS
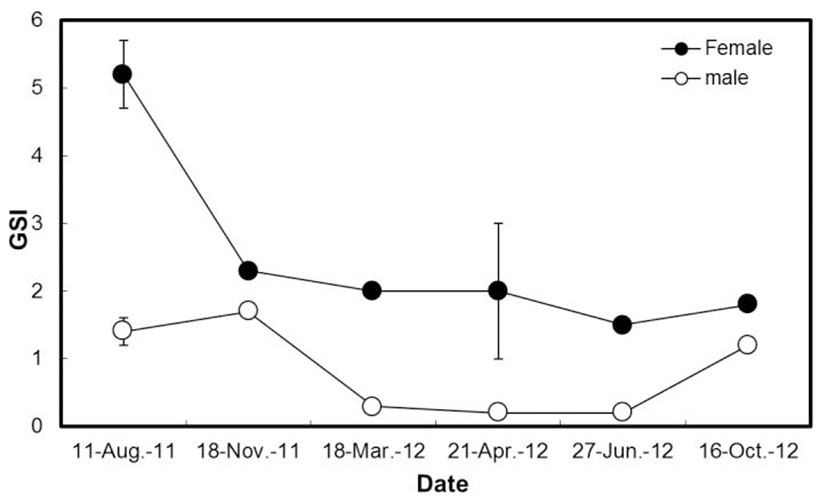
Changes in GSI from August 2011 to October 2012 were depicted in the Fig. 1. The average value of GSI in females was the highest in August 2011 (GSI, 5.2) yet it was lower than after November (GSI, 2.3). On the other hand, in male H. nitens, the GSI reached the highest value in November 2011 (GSI, 1.7) and then gradually decreased; it reached the lowest value from April to June 2012 (GSI, 0.2) but increased up to October 2012 (GSI, 1.2).
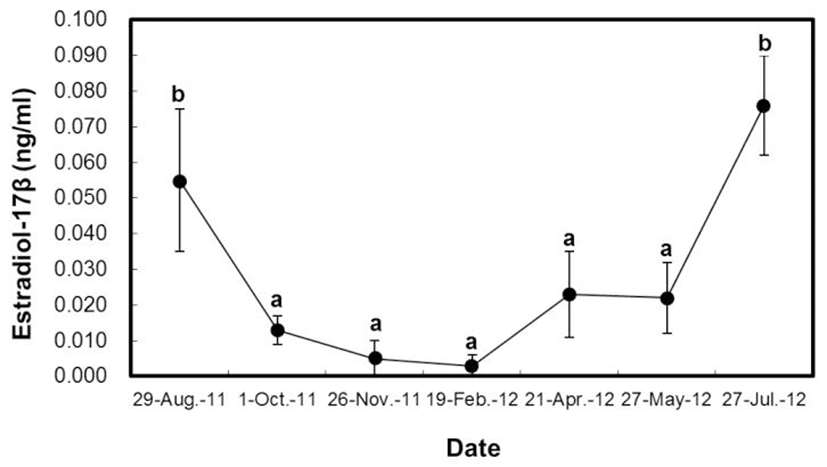
Quantitative changes in plasma hormones of cultured H. nitens were shown in the Fig. 2 and Fig. 3. The level of estradiol-17β in female H. nitens was 0.055 ± 0.020 ng/mL in August 2011 (the spawning period), while it was largely decreased in October 2011 (0.013 ± 0.004 ng/mL), as the period of after spawning period. And then, their values are continuously decreased, and reached the minimum value (0.003 ± 0.003 ng/mL) in February 2012 (the wintering period). Thereafter, it was gradually increased up to the culture period and reached the maximum value (0.076 ± 0.014 ng/mL) in July 2012 (before spawning period). In the present study, no statistical difference can be found in levels of hormones in August 2011 (the spawning period) and July 2012 (before spawning); however, there was a significant difference between October 2011 (after spawning period) and May 2012 (the culture period; P<0.05).
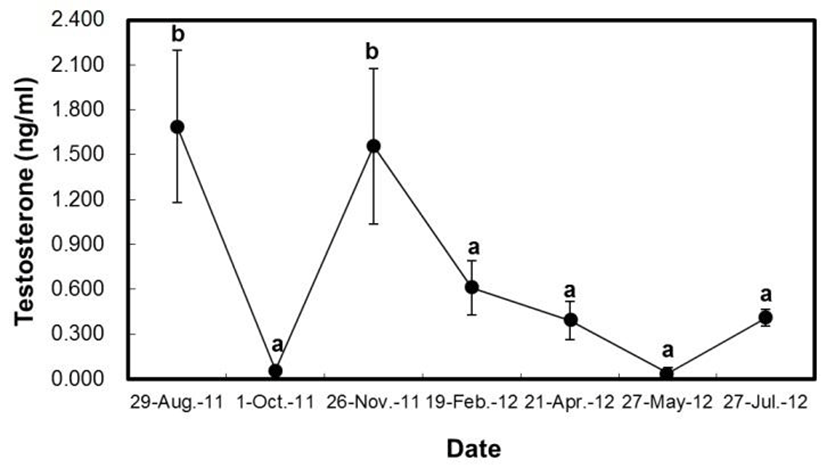
In contrast, in male H. nitens, the level of testosterone was the maximum in August 2011 (1.688 ± 0.511 ng/mL) during the spent period, and then it was drastically decreased in October 2011 (0.058 ± 0.009 ng/mL), after the spent period, which was the lowest level throughout the study. After this, in November 2011, the level of testos-terone was increased (before wintering; 1.556 ± 0.518 ng/mL), and then decreased again in the wintering period (February 2012; 0.610 ± 0.183 ng/mL).
In the culture period (May 2012), the testosterone was shown to be low values as 0.037 ± 0.037 ng/mL, while it was increased in July 2012 (before spent period, 0.407 ± 0.056 ng/mL). In the results herein, there was no differ-rence between the spent period (August 2011) and before the wintering period (November 2011). However, it was statistically different between October 2011 (after the spent period) and February 2012 (the wintering period; P<0.05).
Based on the morphological features and sizes of germ cells and tissue cells around them by histological characteristics, ovarian developmental stages in ovaries in female H. nitens can be classified into five successive stages: early growing, late growing, mature, ripe and spawning, and recovery and resting stages. However, testicular developmental stages in testes in male H. nitens can be divided into four stages: growing, mature, ripe and spent, and recovery and resting stages.
During the period of wintering in late-March (water temperature, 11°C), female individuals were heating cultured. At this time, ovarian development was relatively weak in the early growing stage: it was specifically characterized with chromatin nucleolus oocytes and perinucleolar oocytes of the primary growth stage in the ovarian lobules. These oocytes were 20.4~67.8 μm in diameter, and also a few cortical alveolar oocytes of the primary growth stage were found whose size were 87.8~155.9 μm in diameter (Fig. 4A).
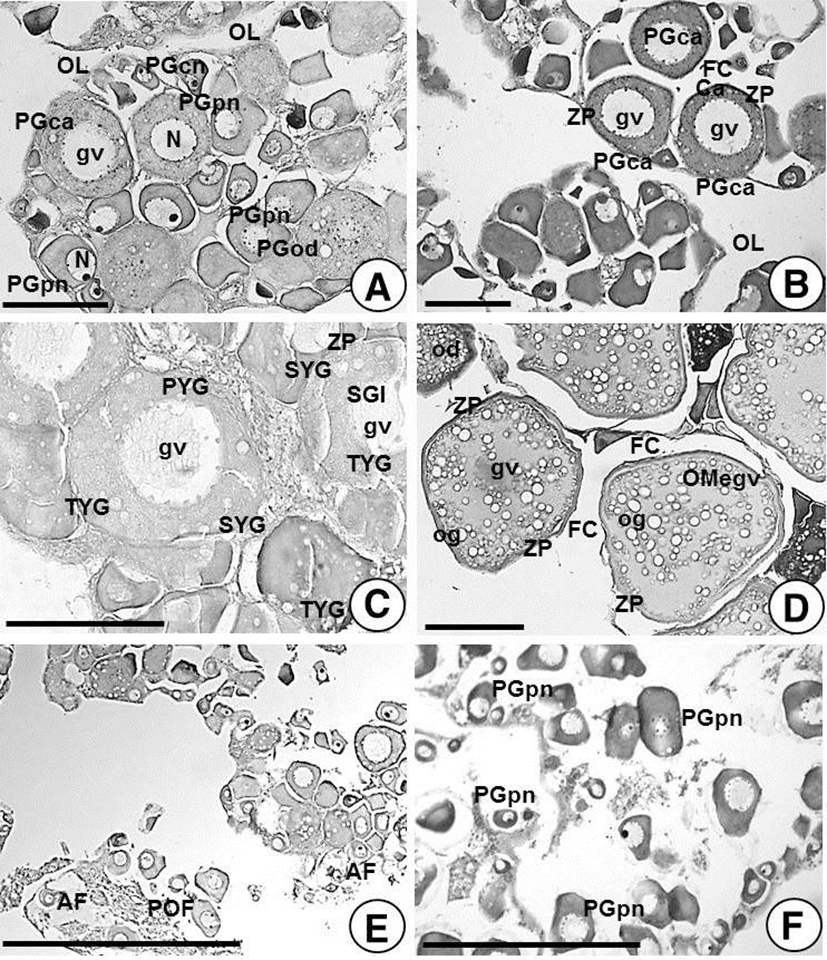
After wintering, natural sea water was used for culturing in late-April (water temperature, 13.0°C). At this stage, ovarian development in ovarian lobules was remarkably developed. In particular, chromatin nucleolus oocytes (20.4~22.3 μm in diameter) and perinucleolar oocytes (60.5~70.8 μm in diameter) of the primary growth stage as well as further developed cortical alveolar oocytes (or yolk vesicle oocytes) in the late growing stage; in this stage, the numbers of oocytes were relatively high yet their diameters were small, stained with dark blue color by hematoxylin staining. In contrast, germ cells were found in the early growing stage were shown to be more developed early secondary growth stage oocytes; in these oocytes, diameters of early yolked oocytes of 115.2~165.5 μm contained oil droplets (Fig. 4B). However, in late-June (water temperature 22.4°C), late yolked oocytes (210.3~252.7 μm in diameter) containing oil droplets, second and tertiary yolk globules of the late secondary growth stage were found in ovarian lobules; the zona pellucida is located in the outer membrane of the oocyte, and the follicle cells are located on the outer layer of the zona pellucida (Fig. 4C).
Female H. nitens, which found between late-July (water temperature 26.5°C) and mid-August (water temperature 25.5°C), are before the spawning period and a number of full-grown oocytes (369~459 μm in diameter) appeared in the ovarian lobules, as well as some of early mature oocytes; in these full-grown oocytes, secondary and tertiary yolk granules (or globules) appeared in the ooplasm homo-genized. In this stage, germinal vesicles of oocytes were shrunk, and moved to the animal pole, and then they disappeared. Around yolk globules, there are multiple oil globules, and a number of small oil droplets were found in all around ooplasm. Ooplasms of mature eggs were acidophil as stained with eosin (Fig. 4D).
In late-August (water temperature, 25.5°C), fully riped eggs (or full-grown oocytes) began to start ovulation from ovarian lobules. In mid-October (water temperature, 25.5°C), postovulatory follicles and atretic follicles were observed in ovarian lobules with residual traces. Ooplasms of chromatin nucleolus and perinucleolar oocytes (approximately 42.8~70.9 μm in diameter), which were found in the primary growth stage, became non-basophilic, and decolored (Fig. 4E).
After spawning, in mid-November (water temperature 11.4°C), ovarian lobules were degenerated/shrunk up to two months from the spawning period; chromatin nucleolus and perinucleolar oocytes (23.5~67.8 μm in diameter) of the primary growth stage were found in ovarian lobules with decolored basophilic cytoplasm (Fig. 4F).
In testicular lobules of male H. nitens found between late-June (water temperature 22.4°C) and mid-July (water temperature 25.4°C), spermatogenesis began to start; spermatocytes, spermatids and sperms during spermiogenesis were found in testicular lobules (Fig. 5A).
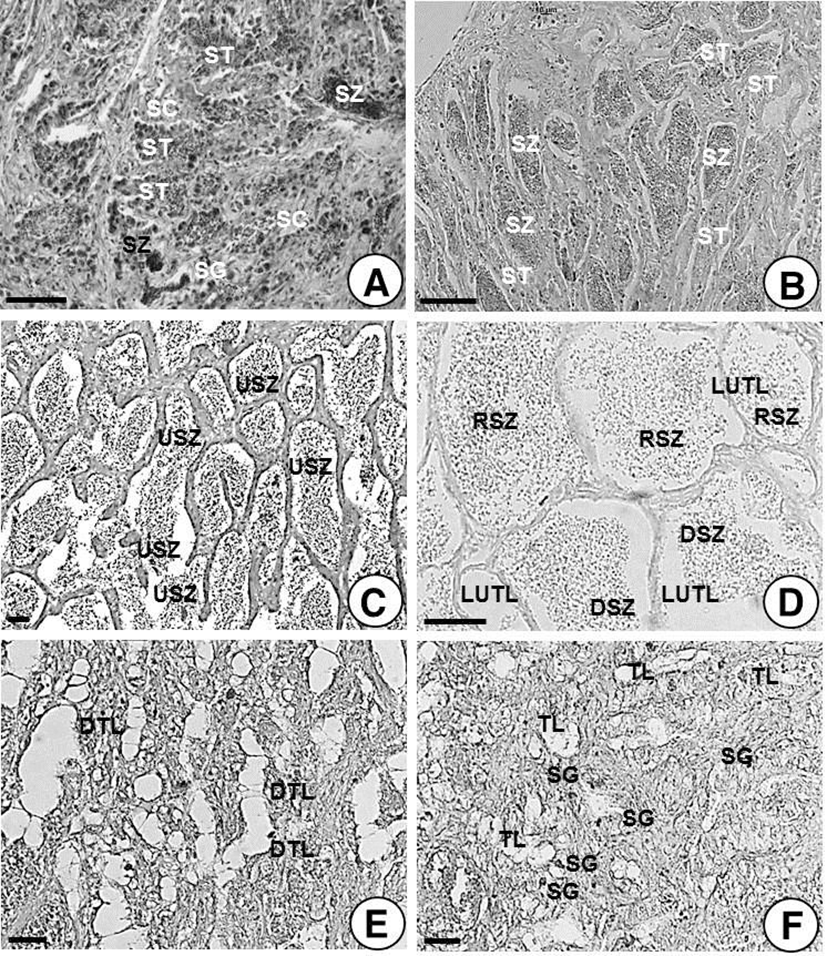
Most male H. nitens found between late-July (water temperature 26.5°C) and mid-August (water temperature 25.5°C) contained many testicular lobules, which were filled with a number of spermatozoa and spermatids as well as small number of spermatocytes (Fig. 5B).
Between late-August (water temperature 25.5°C) and mid-October (water temperature 20.4°C), male H. nitens contained many testicular lobules, which were fully filled with undischarged sperms in the ripe and spent stage (Fig. 5C). After mid-November (water temperature 11.4°C), most fishes were after spent stage. At this time, testicular lobules contained remaining undischarged sperms which were in the center of lobules; a number of sperms were degenerated while edges of lumen of testicular lobules were shown to be empty as most sperms were discharged (Fig. 5D).
While wintering, between late-March (water temperature 11.5°C) and early-April (water temperature 13.0°C), most male H. nitens had degenerated or shrunken testicular lobules in the testes. Therefore, it was difficult to find their original shapes as well as germ cells (Fig. 5E). Even after wintering, from the resting stage to late April (water temperature 13.1°C), testicular lobules were alike as shown previously. In this, a few spermatocytes were found and monitored the early stage of spermatogenesis (Fig. 5F).
DISCUSSION
H. nitens is one of the subtropical species and has been gaining much attention for fish farming as they are frequently harvested in both Southern and Western coast of Korea possibly due to elevated water temperature from global warming (Lim & Choi, 2009). Given its significance as a novel aquaculture species, it is timely and important to elucidate biological factors as well as accurate gonad developmental stages of H. nitens in indoor culture for the species conservation as well as development of aquaculture technology. To date, regarding our knowledges of this species, little information is available with regards to artificial indoor culture and breeding ecology of H. nitens hence studies about their maturation and spawning are significant.
Most teleostean fishes are matured, and spawn in certain periods; there is periodicity for developmental changes of intra-structures of reproductive organs, mainly around their spawning stage. These periodical changes are known to be modulated by various hormones secreted from endocrino-logical system which can be stimulated via multiple environmental factors such as water temperature as well as photoperiod (Stressmann et al., 1996; Kang et al., 2004b; Kang et al., 2008; Kang et al., 2012).
Generally, the GSI is calculated in order to indirectly estimate the spawning period. Changes in the GSI of cultured female H. nitens in the landbased fish culture tank was found to be the highest in August (GSI, 5.2) while male H. nitens had the highest value in November (GSI, 1.7) followed by gradual reduction which was similar results demonstrated using wild H. nitens in which fishes were induced for natural spawning in between August and September (Kang et al., 2004a).
Trends of changes in plasma estradiol-17β of female H. nitens were somewhat in agreement with development of oocytes and GSI changes. It has been known that estradiol-17β modulates formation of vitellogenin (Nagahama, 1987). In the study, we found that this hormone was gradually increased from July when vitellogenin made for H. nitens followed by reduction in early-October (after spawning stage) which was also demonstrated in H. otakii (Lee et al., 2000). Fish testes secret steroid hormones in response to stimulation of GTH produced and secreted by the pituitary gland. Of these steroid hormones, it has been reported that testosterone is produced from steroid hormone producing of Leydig cells of interstitial tissue and involved in spermatogenesis as well as secondary sexual characteristics development (Nagahama et al., 1998). The level of testosterone in male H. nitens was shown to be highest before and after the spent stage which is in agreement with spotlined sardine, Sardinops melanostictus (Matsuyama et al., 1991), and H. otakii (Lee et al., 2000).
In our study, the ovarian development of H. nitens was getting into the ripe-spawning stage in between mid-August and September as the maximal GSI value was found in this period. In the recovery and resting period, after mid-November, ovarian lobules are degenerated and shrunk so that basophilic chromatin nucleolus and peri-nucleolar oocytes were observed. In the recovery and resting period, as demonstrated in other studies [e.g., T. obscurus (Kang et al., 2008)], RNA is extruded into cytoplasm of chromatin nucleolus and perinucleolar oocytes thereby representing dark blue color in an optical micro-scope upon staining with hematoxylin. In the testis of H. nitens, a number of testicular lobules are present; in their mature stage, in mid-August, most male H. nitens have testicular lobules filled with sperms. From the wintering period to late-April, testicular lobules were degenerated and shrunk as the recovery and resting stages are main-tained for a while. In mid-October, right after spent sperms, all testicular lobules were filled with sperms.
Taken altogether, in the present study, the authors studied the artificial indoor cultures and breeding of H. nitens via investigating gonadal development and changes in plasma sex hormones; these results herein are expected to be utilized as an important preliminary data for artificial breeding of H. nitens.

