INTRODUCTION
The primary function of the uterus is supporting fertility, and the endometrium is the layer critically involved in receiving an embryo, facilitating implantation and decidualization, and supporting embryo growth and development until placentation (Marquardt et al., 2019). The uterus is a target tissue of ovarian steroid hormones. Estradiol (E2) stimulates endometrial proliferation and growth, while progesterone (P4) suppresses E2 induced epithelial cell proliferation, concomitant with initiation of stromal cell differentiation (Marquardt et al., 2019). These dynamic changes are necessary for embryo implantation and successful pregnancy (Tabibzadeh, 1998). The endometrium undergoes well-defined and regulated gene expression in preparation for implantation (Fox et al., 2016).
The physiological effects of P4 are mediated by the progesterone receptor (PGR). P4 and PGR together play a central role in reproductive events associated with the establishment and maintenance of pregnancy. PGR regulates implantation, decidualization, and glandular development via a complex paracrine signaling network (Wetendorf & DeMayo, 2014; Adams & DeMayo, 2015; Bhurke et al., 2016). The pivotal role of PGR within reproductive tissues causes it to be an area of focus within reproductive diseases (Teasley et al., 2018). Specifically, reduced expression of PGR has been associated with endometriosis, a prevalent disease in women’s health (Grandi et al., 2017). Overall, aberrations of steroid hormone signaling can be found in many uterine disorders including infertility, endometriosis, endometrial cancer, and uterine leiomyoma (Han & O’Malley, 2014; Islam et al., 2017; Patel et al., 2017; Yoo et al., 2017).
AT-rich interaction domain 1a (ARID1A) is one subunsit within the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling complex (Chunder et al., 2003). It is located in chromosomal region 1p36, which is a region that is often deleted within many different forms of cancer (Chunder et al., 2003; Takeda et al., 2016). A high frequency of mutation of ARID1A in cancers, specifically endometrioid cancer of the uterus and ovarian clear cell and endometrioid cancer, has revealed the potential of ARID1A to be defined as a tumor suppressor (Jones et al., 2010; Guan et al., 2011a). ARID1A is able to inhibit tumor growth and cellular proliferation (Guan et al., 2011b). Several studies have linked SWI/SNF and ARID1A to transcriptional regulation, particularly nuclear hormone-induced transcription and expression of cell cycle regulators (Mao & Shih, 2013; Samartzis et al., 2013; Wu & Roberts, 2013). Previously, we have shown that ARID1A protein levels are lower during both the proliferative and secretory phases, in epithelial and stromal cells of women with endometriosis compared to those without (Kim et al., 2015). Ablation of Arid1a in the murine uterus results in the inability to inhibit E2-induced epithelial cell proliferation and E2-responsive target gene expression (Kim et al., 2015). However, the molecular mechanism of ARID1A action in steroid hormone regulation and pregnancy is not well studied.
Proline-rich acidic protein 1 (PRAP1) is a secretory protein of 149 amino acids, with a 20 amino acid signal peptide at its N-terminal. PRAP1 has been identified as a differentially expressed gene in the pregnant uterus of rodents (Kasik & Rice, 1997). It is strongly expressed in the late pregnant uterus but disappears from the uterus within 3 days after birth (Kasik & Rice, 1997). Human PRAP1 mRNA is detected in the gastrointestinal tract, liver, and kidney. PRAP1 is localized in the epithelial lining of the colon and cervix, the hepatocytes, and the kidney proximal and distal tubules (Zhang et al., 2003). PRAP1 plays an important role in maintaining normal growth homeostasis in epithelia (Lanemo Myhrinder et al., 2008; Zhang et al., 2003). PRAP1 is a p53-responsive gene induced by genotoxic stress (Huang et al., 2012). It is an interacting partner of MAD1 and has a suppressive role in mitotic checkpoint signaling in hepatocellular carcinoma (Sze et al., 2014). Prap1 mRNA is highly expressed in the uterine luminal epithelium of gestation day (GD) 0.5 in mice, disappears in the preimplantation day 3.5 uterus, and reappears abundantly in the luminal epithelium after embryo implantation (Diao et al., 2010). Therefore, PRAP1 is suggested as a marker for successful embryo implantation (Diao et al., 2010).
While Prap1 is up-regulated by E2, it is down-regulated by P4 (Diao et al., 2010; Xiong et al., 2011). However, the regulation of PRAP1 in the uterus is still elusive. In order to explore the regulation of PRAP1 expression in the uterus, we investigated the expression of PRAP1 in the uterus of Pgr knockout (PRKO) and uterine specific Arid1a knockout (Pgrcre/+Arid1af/f; Arid1ad/d) mice. We also evaluated the regulation of PRAP1 in response to steroid hormones in the uterus.
MATERIALS AND METHODS
All mouse experiments were approved by the Institutional Animal Care and Use Committee of Michigan State University (IACUC number: 11/16-192-00). For the uteri samples during early pregnancy, wild C57BL/6 female mice at 8 weeks of age were individually mated with wild-type male mice and uteri were collected at different time points of pregnancy. The initiation of pregnancy was marked by the presence of the postcoital vaginal plug as day 0.5 of gestation (GD 0.5) for early pregnancy study. In order to investigate the expression of PRAP1 by PGR and ARID1A in the uterus, we used PRKO and Arid1ad/d mice (Lydon et al., 1995; Kim et al., 2015). We previously generated mice with conditional ablation of Arid1a in the PGR positive cells (Arid1ad/d) to study the role of Arid1a in the uterus (Kim et al., 2015). Uterine tissues were collected from both horns then were stored at −80°C for RNA or fixed in 4% paraformaldehyde (vol/vol) and paraffin embedded.
To study PRAP1 expression by steroid hormone regulation, wild-type C57BL/6 mice, Arid1ad/d or PRKO mice at 6 weeks of age underwent bilateral ovariectomy. After at least 2 weeks to eliminate endogenous ovarian hormone completely, the mice were given subcutaneous injection with vehicle (sesame oil) or P4 (1mg/mouse) (n=3 per genotype per treatment). The uteri were collected at 6 hours after the steroid hormone injection.
To study PRAP1 expression by steroid hormone regulation, wild-type C57BL/6 mice, Arid1ad/d or PRKO mice at 6 weeks of age underwent bilateral ovariectomy. After at least 2 weeks to eliminate endogenous ovarian hormone completely, the mice were given subcutaneous injection with vehicle (sesame oil) or P4 (1mg/mouse) (n=3 per genotype per treatment). The uteri were collected at 6 hours after the steroid hormone injection.
Total RNA was isolated from uteri using Qiagen RNeasy total RNA isolation kit (Qiagen, Valencia, CA, USA). The expression levels of Prap1 were quantified by RT-qPCR using an Applied Biosystems StepOnePlus system according to the manufacturer’s instructions (Applied Biosystem, Foster City, CA, USA). The cDNAs were synthesized with MMLV Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) by the use of 1 μg of total RNA primed with random hexamer primers according to the manufacturer’s instructions. RT-qPCR was performed on cDNA to assess the expression levels of genes of interest with primers, by using SYBR green and 96-well optical plates, with an Applied Biosystems StepOnePlus (Applied Biosystem, Foster City, CA, USA). Experimental Prap1 data were normalized to 18S ribosomal RNA. Analysis of Prap1 mRNA expression was first undertaken by the standard curve method, and results were corroborated by CT values assessing levels of gene expression. All data are presented as mean ± SEM. Statistical analyses were performed using Student’s t-tests using the Instat package from GraphPad (San Diego, CA, USA). p<0.05 was considered statistically significant.
Uterine sections of 5μm thickness were blocked with 10% normal goat serum in PBS (pH 7.5) for immunohistochemistry. Sections were exposed to primary anti-PRAP1 (HPA038713, Sigma-Aldrich, St. Louis, MO, USA) antibody in 10% normal goat serum in PBS (pH 7.5) overnight at 4°C. The Sections were incubated with biotinylated goat anti-rabbit 2nd antibody (BA-1000; Vector Laboratories, Burlingame, CA, USA). Following exposure to the horseradish peroxidase-conjugated streptavidin substrate, positive immunoreactivity (brown precipitate) was detected using the Vectastain Elite DAB kit (Vector Laboratories, Burlingame, CA, USA) and hematoxylin (Biocare Medical, Pacheco, CA, USA) were used for a nuclear counterstain.
RESULTS
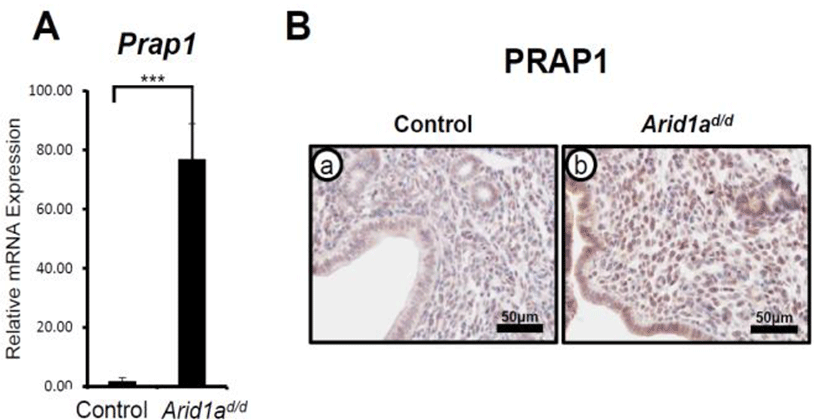
ARID1A has a critical role in modulating epithelial proliferation at the pre-implantation stage which is a critical requisite for uterine receptivity (Kim et al., 2015). To determine transcriptional regulation of Prap1 by ARID1A, RT-qPCR was performed in the uteri of control (Arid1af/f) and Pgrcre/+Arid1af/f (Arid1ad/d) mice (Kim et al., 2015) at GD 3.5. The results revealed that there was a significant increase in Prap1 mRNA expression in the Arid1ad/d mice uterus compared to the control mice (Fig. 1A). This result was extended through immunohistochemistry to examine spatial expression of PRAP1 protein in the Arid1ad/d mice (Fig. 1B). Control samples at GD 3.5 showed very weak PRAP1 expression within the uterine epithelial, glandular, and stromal cells. However, Arid1ad/d mice showed a remarkable increase in PRAP1 expression throughout when compared to control mice. These data suggest that the expression of PRAP1 is suppressed by ARID1A.
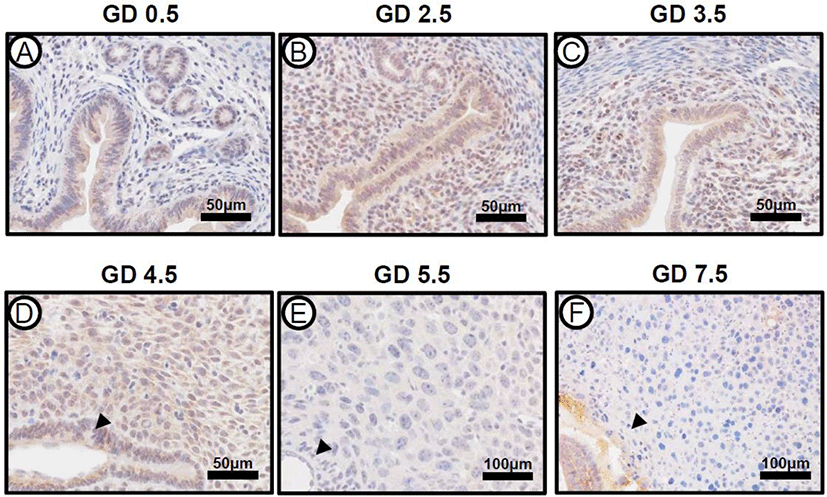
The expression of ARID1A mRNA and protein was strongly detected on GD 0.5, which consistently expressed until GD 6.5 in the uterus (Kim et al., 2015). To investigate the expression pattern of PRAP1 in mouse uteri during early pregnancy, we performed immunohistochemistry from GD 0.5 to GD 7.5 in the uteri of natural pregnancy (Fig. 2). The initiation of pregnancy was marked by the presence of the postcoital vaginal plug (GD 0.5). At GD 0.5, PRAP1 expression was present within uterine luminal and glandular epithelium, however expression was not seen within stroma. At GD 2.5, expression increased, and was also present within stroma. At GD 3.5 and 4.5, PRAP1 levels showed a slight decrease in uterine epithelial and stromal cells. Interestingly, the expression of PRAP1 was remarkably decreased after embryo implantation GD 5.5 and 7.5. The expression of PRAP1 was not observed in the uterus including the primary and secondary decidual zone at GD 5.5 and 7.5. These data indicate that PRAP1 is tightly regulated in the uterus during early pregnancy.
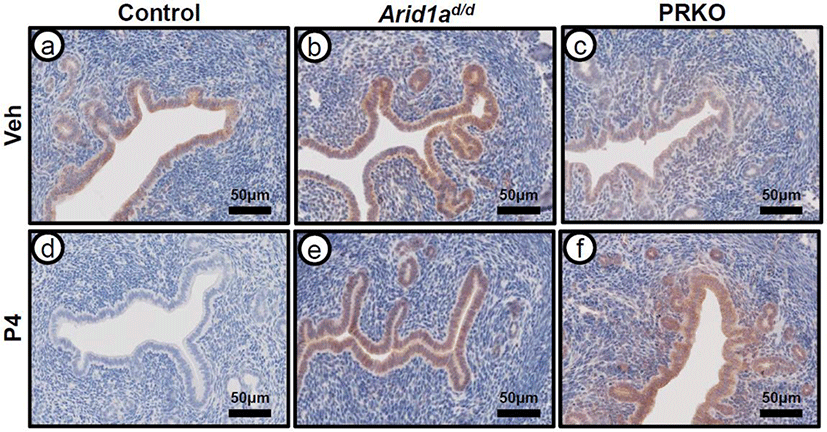
In order to evaluate PRAP1 expression in response to P4 treatment, immunohistochemistry was performed on ovariectomized control (wild type and Arid1af/f), Arid1ad/d, and PRKO mice treated with either vehicle or P4 for 6 hours (Fig. 3). PRAP1 expression was strongly detected at luminal and glandular epithelium of the ovariectomized control mice treated with vehicle. After P4 treatment, the strong PRAP1 expression disappeared at the luminal and glandular epithelium of control mice. Both Arid1ad/d and the PRKO mice showed expression of PRAP1 after vehicle treatment. However, PRAP1 expression was not changed in Arid1ad/d and PRKO mice after P4 treatment. This data further reveals that PRAP1 is regulated by PGR and ARID1A.
DISCUSSION
Our study examined the regulation of Prap1 by ARID1A and determined the expression patterns of PRAP1 in the uterus during early pregnancy and in response to ovarian steroid hormone treatment. In the present study, we report that PRAP1 expression levels are remarkably increased in mice with conditional ablation of Arid1a (Arid1ad/d) (Fig. 1). ARID1A plays a role as a transcriptional repressor in breast cancer (Guo et al., 2018). Loss of ARID1A profoundly alters histone modifications and the transcriptome. In response to a lack of Arid1a, mice did not suppress the expression of PRAP1 by P4, further confirming ARID1A is a transcriptional suppressor of Prap1 gene.
Throughout implantation and early pregnancy, fluctuations in steroid hormone expression are exhibited. GD 0.5 and 1.5 in murine pregnancy are the days in which pre-ovulatory ovarian E2 is secreted, and proliferation occurs. From GD 2.5 on, proliferation shifts from the luminal and glandular epithelial cells to the stromal cells. Overall, we observed fluctuation of PRAP1 expression (Fig. 2). At GD 2.5 expression levels were the highest overall. E2 stimulated proliferation occurs during the first two days of pregnancy in the mouse uterus (Das, 2010). Our result is consistent that PRAP1 is regulated by E2 (Xiong et al., 2011). At GD 4.5, implantation occurs, as stromal cells transition into decidual cells in response to a blastocyst (Huet-Hudson et al., 1989). We and others showed reduction of PRAP1 expression after implantation and down-regulation by P4 (Diao et al., 2010; Xiong et al., 2011). The invading blastocyst then induces a decidualization reaction of the P4-primed stromal cells, where they differentiate into morphologically and functionally unique cells to surround the implanting embryo and support growth until placentation, all under critical continued P4 regulation (Marquardt et al., 2019). Therefore, PRAP1 is a suppressed protein at the post-implantation stage.
PRKO is an invaluable model in assessing P4’s role in gene and protein expression. Past research has revealed that the ablation of PGR results in abnormalities in the reproductive biology of the mouse and also a defect in the implantation process (Vasquez & DeMayo, 2013). Within this study, we utilized the PRKO mouse model in order to analyze the expression of PRAP1 in response to the ablation of both PGR isoforms (Fig. 3). Results showed a difference in only epithelial expression of PRAP1 in the PRKO mouse model in comparison to controls. Epithelial cells are associated with the production of paracrine factors, mediated through the PGR receptors, which are critical in enhancing epithelial differentiation and growth within the uterus (Lee et al., 2006; Franco et al., 2012). These results suggest that epithelial PRAP1 expression is regulated by PGR.
P4 is a steroid hormone that is critical in reproductive processes, and has been utilized for therapeutic treatment in peri-menopausal women who experience aberrant bleeding or menstrual problems (Prior, 2011; Czyzyk et al., 2017). In order to analyze P4’s effect on PRAP1 expression, we examined PRAP1 expression within control, Arid1ad/d, and PRKO mice, with both vehicle and 6-hour P4 treatment (Fig. 3). In this analysis we found that P4 treatment decreased expression in the control mice. This result allows us to hypothesize P4’s role in PRAP1 expression, and also allows for a connection of P4 treatment in the two different mouse models. P4 does not suppressed PRAP1 expression in the Arid1ad/d mice. PRAP1 plays normal epithelial cell homeostasis as a negative regulator and the down-regulation of Prap1 in cancer cells may lead to dysregulated cell growth (Zhang et al., 2003). Although the regulation of PRAP1 expression by ovarian steroid hormone suggests a possible connection to uterine function, molecular mechanisms of PRAP1 regulation should be dissected in uterine function during early pregnancy.
Our results demonstrate that PRAP1 is a novel target of ARID1A and PGR in the murine uterus. Additional research within this context would provide for greater insight into the role of PRAP1 within the context of reproductive biology, and provide for a new possible therapeutic target for uterine disorders.

