INTRODUCTION
The uterus is an important hormone-responsive tissue in the female reproductive system. Histologically, the uterus is comprised of three layers, including the perimetrium, myometrium, and endometrium. Among them, the endometrium has an essential function in embryo implantation and development to achieve successful gestation. It is the innermost layer of the uterus which consists of both luminal and glandular epithelial and stromal cells. In mammals, the uterine endometrium undergoes a dynamic cyclical growth and tissue remodeling controlled by the ovarian hormones, estrogen, and progesterone during the menstrual (human) and the estrous (rodent) cycle (Evans et al., 1990; Dharma et al., 2001; Wood et al., 2007). This dynamic remodeling involves the recurrent synchrony of cell proliferation, differentiation, and apoptosis, along with extracellular matrix (ECM) turnover, leukocyte infiltration, and angiogenesis (Evans et al., 1990). Estrogen is usually known to induce endometrium growth by thickening the mucosa during the follicular phase (Groothuis et al., 2007). On the other hand, progesterone blocks estrogen-induced endometrial growth and transforms it into a receptive state for blastocyst implantation. Classically, estrogen regulates the expression of an array of genes to mediate its major actions on uterine cells via its nuclear receptors, ERα and ERβ. On the other hand, progesterone regulates its actions on the uterine via progesterone receptor (PR), PR-A, and PR-B in the uterus (Mulac-Jericevic & Conneely, 2004).
Transcriptional coactivator with PDZ-binding motif (TAZ) was first discovered as a phosphoprotein, which interacts with 14-3-3 proteins (Kanai et al., 2000). TAZ shares high similarity in structural and functional parts of Yes-associated protein (YAP) (Piccolo et al., 2014). The activity of TAZ is regulated by nuclei-cytoplasmic localization in a phosphorylation-dependent manner of the upstream kinases, including mainly LATS1/2 which is known to be tightly controlled by the Hippo signaling pathway (Lei et al., 2008). In a classical view, if the Hippo signaling turns on, Ste20-like kinase 1 (MST1; also known as STK4) and Ste20-like kinase 2 (MST2 also known as STK3) are auto phosphorylated and activate large tumor suppressor kinase 1 (LATS1) and LATS2. Activated LATS1/2 induces the phosphorylation of YAP/TAZ, which sequesters or degrades them in the cytoplasm thereby inhibiting their transcriptional co-activator function (Zhao et al., 2010). When the Hippo signaling turns off, YAP/TAZ are dephosphorylated and translocated in the nucleus. YAP/TAZ, which lacks DNA-binding sites, functions as a coactivator via its intrinsic transactivation domain which interacts with several transcription factors, mainly the TEAD family in the nucleus (Mahoney et al., 2005; Zhang et al., 2009). Several studies have shown that Hippo signaling is regulated by several intracellular and extracellular signals that involve cell junctions, cell polarity, mechanical forces, and several cell membrane receptors such as G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) (Yin et al., 2013). It is also known to collaborate with Wnt signaling (Azzolin et al., 2012).
The biological function of TAZ is usually involved in various physiological cellular processes, including cell proliferation, migration, apoptosis, differentiation, and senescence (Varelas et al., 2008; Di Palma et al., 2009; Jeong et al., 2010; Wang et al., 2016; Kim et al., 2019) in particular, many studies reported that TAZ is an oncogenic protein that promotes the multiple gene expression involved in tumorigenesis thereby inducing the self-renewal of stem cells, promoting cell proliferation, cell migration and epithelial to mesenchymal transition (EMT) (Chan et al., 2008; Lei et al., 2008; Zhang et al., 2009). Consistently, multiple cancers including invasive ductal breast cancer, glioblastoma, lung adenocarcinoma, ovarian cancer, and endometrial cancer are associated with an elevated level of TAZ expression (Chan et al., 2008; Bhat et al., 2011; Zhou et al., 2015).
According to recent studies, TAZ expression was associated with the most aggressive forms of endometrial carcinoma and its overexpression promoted the EMT related factors, leading to an increase in the migratory and invasive capacity of endometrial cancer cell lines (Romero-Perez et al., 2015). Also, high expression of TAZ was associated with a high clinical stage in patients with endometrioid adenocarcinoma (Zhan et al., 2016). Moreover, TAZ expression was increased in pregnant and decidual mice uteri compared to non-pregnant mice and its overexpression induced proliferation in decidual endometrial stromal cells (ESCs; Yu et al., 2021). Finally, estrogen promoted breast cancer cell proliferation, migration, and tumorigenesis by activating and stabilizing TAZ protein via the G protein-coupled estrogen receptor (GPER) (Zhou et al., 2015). As such, several studies suggest the possibility that TAZ may be associated with ovarian steroid hormones, particularly estrogen in the endometrium. But it is not yet clear whether TAZ expression is regulated by ovarian steroid hormones in the uterus. Therefore, we investigated the expression pattern of TAZ during the estrous cycle in a normal mouse uterus and the regulation of TAZ by estrogen and progesterone in the OVX mouse uterus.
MATERIALS AND METHODS
All mice experiments were performed on 7-week-old ICR mice provided by JA BIO (Suwon, Korea). Mice were housed and fed ad libitum under temperature- and light-controlled conditions with lights on for 12 h daily. Animal care and use were performed following the guidelines for the Care and Use of Laboratory Animals, and this study was approved by Institutional Animal Care and Use Committee (IACUC, Approval No. KU22074).
The estrous stages were distinguished using the vaginal smear assay as in previous studies (Lee et al., 2021). A little amount (0.1–0.2 mL) of phosphate buffered saline (PBS) was inserted into the entrance of the mouse vaginal and drawn back into the pipette four to five times. Hematoxylin and eosin staining method was used in staining epithelial cells. Collected PBS containing a few drops of cell suspension was expelled onto Histobond® adhesive Slide Glass (Ducksan General Science, Seoul, Korea), dried on a 65°C heat block, and then stained with hematoxylin (Merch, Darmstadt, Germany) for 30 seconds. Slides were rinsed with tap water for 5 min and incubated in 50%, 75%, and 90% ethanol for 5 min. After staining it with eosin Y (Cancer Diagnostics, Durham, CA, USA) for 5 min, the slides were washed in 90% ethanol and 100% ethanol for 5 min. Finally, the slides incubated in xylene were mounted with GEL/MOUTN™ (Biomeda, Foster City, CA, USA). Staining was observed using a microscope, and each stage of the estrous cycle was discriminated by determining the relative numbers of three cell types including nucleated epithelial cells, cornified epithelial cells, and leukocytes. After determining the estrous cycle, the uteri of each stage were collected. Some of the uteri are used for RNA and protein preparation, and the remainder were fixed in 4% paraformaldehyde to be used in making paraffin blocks for immunostaining.
To examine the effects of ovarian steroid hormones on the expression of TAZ in mouse uterus, 7-week-old ICR mice were ovariectomized (OVX) and recovered for 2–3 weeks before E2 and P4 treatment as in previous studies (Moon et al., 2019; Cho et al., 2020). The mice were anesthetized by intraperitoneal injection of (0.4–0.5 mL) 2.5% 2,2,2-Tribromoethanol (Avertin) (Sigma-Aldrich, St. Louis, MO, USA). The mouse skin of the dorsal abdomen was cut, then the ovaries underneath the fat pad were carefully removed. To suture the sclera, sterile suture (ALEE CO, Busan, Korea) was used and the leather was sutured using a wound clip applier (Roboz Surgical Instrument, San Diego, CA, USA). The suture site was disinfected with povidin (Firson, Cheonan, Korea) and mice were placed on a 42°C heat warmer to observe the condition until they woke up from anesthesia. After a 2-week recovery, the OVX mice were treated subcutaneously with β-estradiol (E2, 200 ng/mouse, Sigma-Aldrich) or progesterone (P4, 2 mg/mouse, Sigma-Aldrich). To investigate the time-dependent effects of estrogen and progesterone, uteri were collected at 0, 2, 4, 6, 12, and 24 h after E2 and P4 injection. To determine whether the expression of TAZ in the mouse uterus is dependent on estrogen receptors, an estrogen receptor antagonist ICI 182,780 (500 μg/mouse, Medchemexpress, Princeton, NJ, USA) was pretreated 30 min before estrogen treatment (Moon et al., 2019). Sesame oil (100 μL/mouse, Acros Organics, Geel, Belgium) was used for control mice. Mice were sacrificed and uteri were collected for histological analyses, RNA, and protein extraction.
Uteri were collected from mice and immediately frozen in liquid nitrogen. Total RNAs were extracted from uteri using RNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer’s instruction. After RNA preparation, potential genomic DNA was digested with DNase I (RNase-free) (NEB, Ipswich, MA, USA). The total RNA (1 μg) was reverse transcribed to synthesize complementary DNA (cDNA) using SensiFAST™ cDNA Synthesis Kit (Meridian Bioscience, Cincinnati, OH, USA) according to the manufacturer’s instruction. RT-PCR was performed using the Proflex PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The PCR temperature cycling conditions were as follows: initial denaturation for 5 min, followed by 30 cycles: denaturation at 95°C for 30 s, primer annealing at 60°C for 30 s, and extension at 72°C for 20 s. The products were stained with Loading Star (Dyne Bio, Seoul, Korea) and analyzed by gel electrophoresis on 2% agarose gel using Chemidoc™ XRS+ system (Bio-Rad, Hercules, CA, USA). QuantStudio™ 1 Real-Time PCR System (Thermo Fisher Scientific). was used to perform qRT-PCR analysis.
The iQ™ SYBR® Green Supermix (Bio-Rad Life Sciences) was used for amplification and qRT-PCR conditions were as follows: 40 cycles of denaturation at 95°C for 15 s, primer annealing at 60°C for 15 s, and extension at 72°C for 1 min followed by 95°C for 15 s, 60°C for 1 min, and 95°C for 1 s for melt curve. Relative gene expression was calculated by the 2-ΔΔCT method giving the ratios between target genes and a reference gene (Rpl7) (Livak & Schmittgen, 2001). At least 3 animals per experimental group were analyzed by qRT-PCR. Primer sequences for RT-PCR and qRT-PCR are described in Table 1.
Uteri collected from mice were fixed in 4% paraformaldehyde overnight at 4°C for histology and immunostaining. After sufficient dehydration and clearing process, the hardened tissues were embedded in paraffin mold at 60°C and stored at –20°C, until the molten paraffin is sufficiently hardened. Paraffin-embedded uteri were sectioned at a thickness of 5 μm using a microtome (Macroteck, Goyang, Korea) and placed on Histobond® adhesive Slide Glass (Ducksan General Science, Seoul, Korea).
The slides were heated at 60°C–65°C for 5 min to allow the tissue to better adhere to the slides. Then slides were treated two times in xylene for 7 min to be deparaffinized. In the rehydration process, the slides were incubated in 100%, 95%, 70%, and 50% ethanol in the order of once for 5 min, and then finally washed with tap water for 5 min. Next, the slides were boiled in antigen retrieval buffer (10 mM sodium citrate, 0.05% Tween20, ph 6.0) using antigen retrieval steamer (IHC world, Suwon, Korea) for 45 min and cooled at room temperature (RT) for 30 min. Antigen retrieval buffer was washed by incubating the slides one time in tap water and two times in PBS for 5 min. A hydrophobic wall was drawn around the tissues with an ImmEdgepen (Vector Labs, Burlingame, CA, USA) and then incubated with 5% goat serum in 0.3% PBS-T (PBS containing 0.3% Triton X-100) at RT for 2 h. After blocking, the sections were treated with anti-TAZ rabbit polyclonal antibody (1:100 dilution, 4883S, Cell Signaling, Danvers, MA, USA) overnight at 4°C. The primary antibody was washed with 0.1% PBS-T (PBS containing 0.1% tween20) three times for 5 min. Then sections were incubated with a secondary antibody conjugated with Alexa-Fluor antibody 546 (1:500 dilution, A-11010, Thermo Fisher Scientific) for 1 h at RT. The slides were washed with 0.2% PBS-T (PBS containing 0.2% Tween20) three times for 5 min and mounted coverslip with Mounting Medium with DAPI (Abcam, Cambridge, UK). Both primary and secondary antibodies were diluted in 0.1% PBS-T (PBS containing 0.1% tween20). Immunofluorescence analyses were performed using paraffin-embedded uteri obtained from at least 3 animals per experimental group. Finally, slides were observed using a confocal microscope.
Uteri from mice were collected and immediately frozen in liquid nitrogen. Frozen tissues were homogenized with Tissue Lyser LT (Qiagen) in 20 μL/mg RIPA lysis buffer with protease inhibitor cocktail and phosphatase inhibitor mix (NaF 50 mM, Na3VO4 5 mM, β-glycerophosphate 50 mM). Homogenized samples were incubated on ice for 30 min while vortexing every 5 min. After incubation, the samples were centrifuged at 8,000 g for 20 min at 4°C. Following the manufacturer’s instruction, protein concentration was measured by Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific).
A 10–20 μg of total protein was loaded and separated by SDS-PAGE (12% gradient gel) and then transferred to polyvinylidene difluoride membranes (Merck, Darmstadt, Germany). After transfer, the membranes were blocked with 5% DifcoTM Skim milk (BD Biosciences, Franklin, NJ, USA) in 0.1% PBS-T (PBS containing 0.1% Tween20) overnight at 4°C. The membranes were washed in 0.1% PBS-T three times for 5 min and subjected to anti-TAZ mouse monoclonal antibody (1:1,000 dilution, 560235, BD Biosciences) diluted in 4% BSA (Bovogen, Williams, Australia) for 2 h at RT. After three washes with 0.1% PBS-T for 5 min, the membranes were incubated with goat anti-mouse HRP-conjugated antibody (1:10,000, SC-2005, Santa Cruz, Dallas, TX, USA) diluted in 5% skim milk for 1 h at RT. Secondary antibodies were washed with 0.1% PBS-T three times and the blots were developed in Pierce Supersignal Pico ECL substrate (Thermo Fisher Scientific). The chemiluminescence signal was detected with the ChemiDOCTM XRS+system (Bio-Rad) and relative band intensity was quantified using Image J. β-actin antibody (1:10,000 dilution, sc-47778 HRP, Santa Cruz) was used as a loading control on the same blot to normalize the target protein expression. Raw western blot analyses were performed using proteins obtained from at least 3 animals per experimental group (Supplementary Figs. 1 and 2).
All experimental data are reported as mean±SEM. Results were analyzed using one-way ANOVA for statistical evaluation was used for multiple comparisons after the ANOVA in the Statistics Kingdom (https://www.statskingdom.com). For all analyses, p-value less than 0.05 was considered statistically significant.
RESULTS
To investigate the physiological regulation of Taz, we performed RT-PCR analysis using total RNAs from various mouse tissues including the small intestine, stomach, kidney, spleen, liver, heart, brain, lung, ovary, and uterus. The result showed that Taz was highly expressed in the kidney, lung, and ovary (Fig. 1A). This was consistent with previous data (Yue et al., 2014). Interestingly, Taz expression was also high in the uterus compared to other tissues. This suggests that Taz may play an important role in the uterine endometrium.
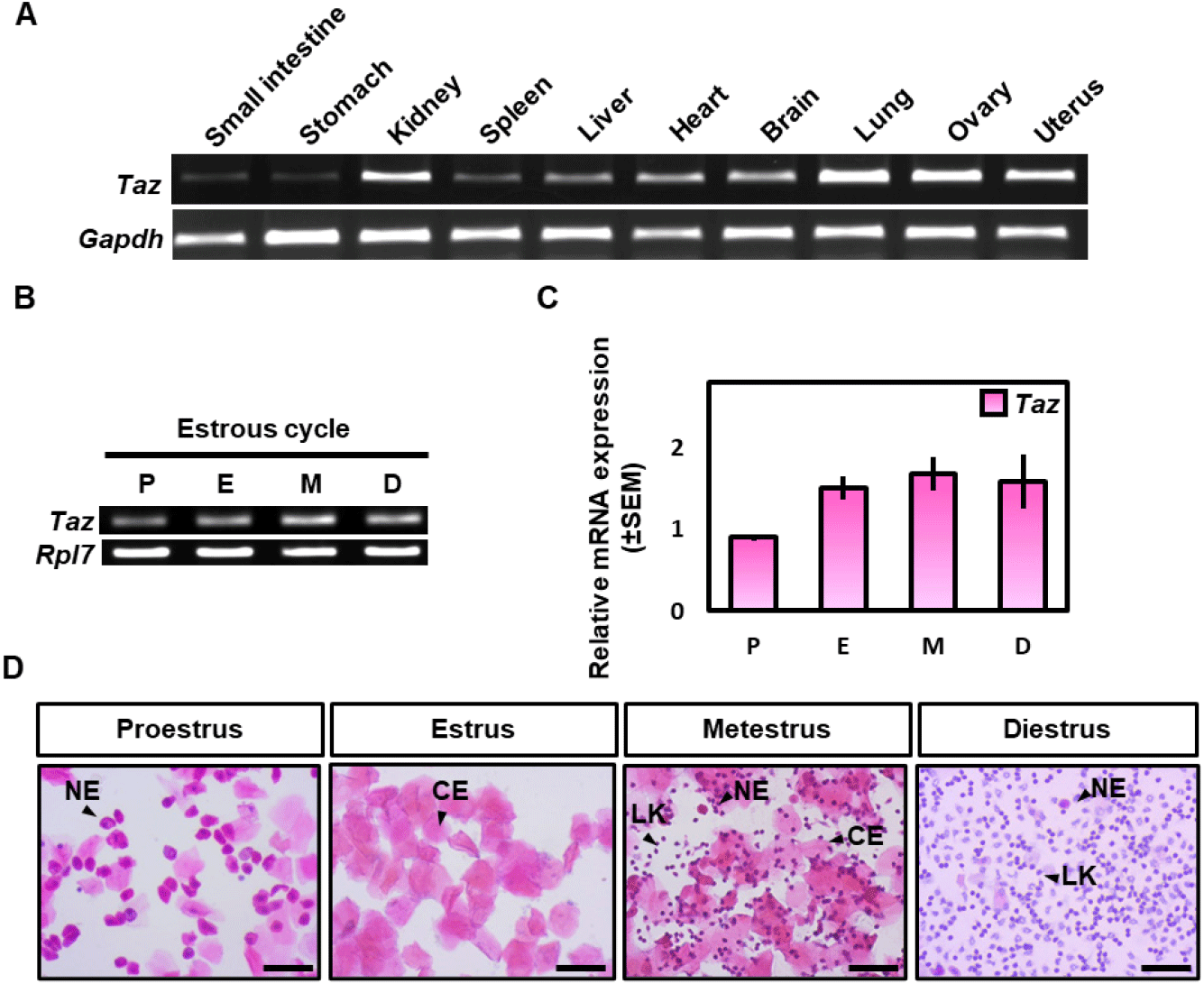
Then RT-PCR and qRT-PCR analysis were performed to examine the expression patterns of Taz in the uterus during the estrous cycle (Fig. 1B and C). A vaginal smear assay was used to differentiate the uterus during the estrous cycle which consists of four stages: proestrus, estrus, metestrus, and diestrus (Fig. 1D). RT-PCR and qRT-PCR analysis showed that Taz mRNA expression was slightly low in the proestrus compared to the other stages, but it didn’t show a statistically significant difference between the estrous cycle. Collectively, these results suggest that the expression of Taz is not significantly regulated according to the estrous cycle.
To investigate the expression level of TAZ protein during the estrous cycle, a western blot analysis was performed. β-actin was used as a loading control to investigate the relative expression level of the TAZ protein. Different from the mRNA result, TAZ expression was a little higher at the estrus compared to the other stages, but there was no statistically significant difference between the estrous cycle (Fig. 2A and B).
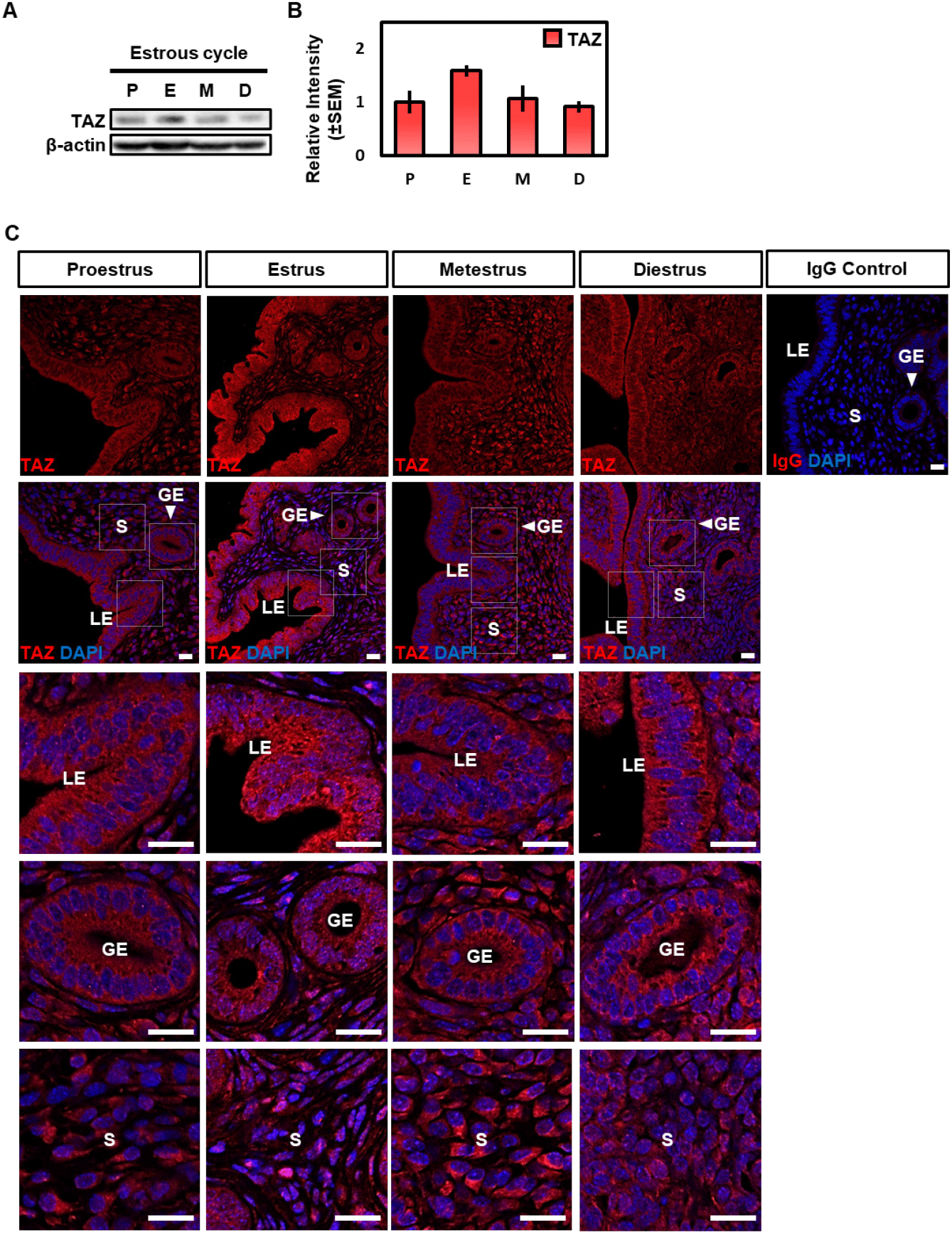
In the next study, we performed immunofluorescence analysis to observe the location of the TAZ protein. Consistent with the western blot result, TAZ expression was slightly high at the estrus and its location was dynamically regulated during the estrous cycle (Fig 2C). TAZ protein was expressed in both epithelial and stromal cells during all stages, but nuclear TAZ expression, especially in the luminal epithelium (LE) was highest in the estrus compared to other stages (Fig. 2C). Collectively, these results suggest that TAZ protein is involved in the morphological and functional dynamics that occur during the estrous cycle through nuclear-cytoplasmic translocation.
Two major ovarian steroid hormones, estrogen, and progesterone regulate dynamic morphological changes in the uterus during the estrous cycle. Therefore, the OVX mouse model was used to investigate the effect of these hormones on TAZ expression in the uterus.
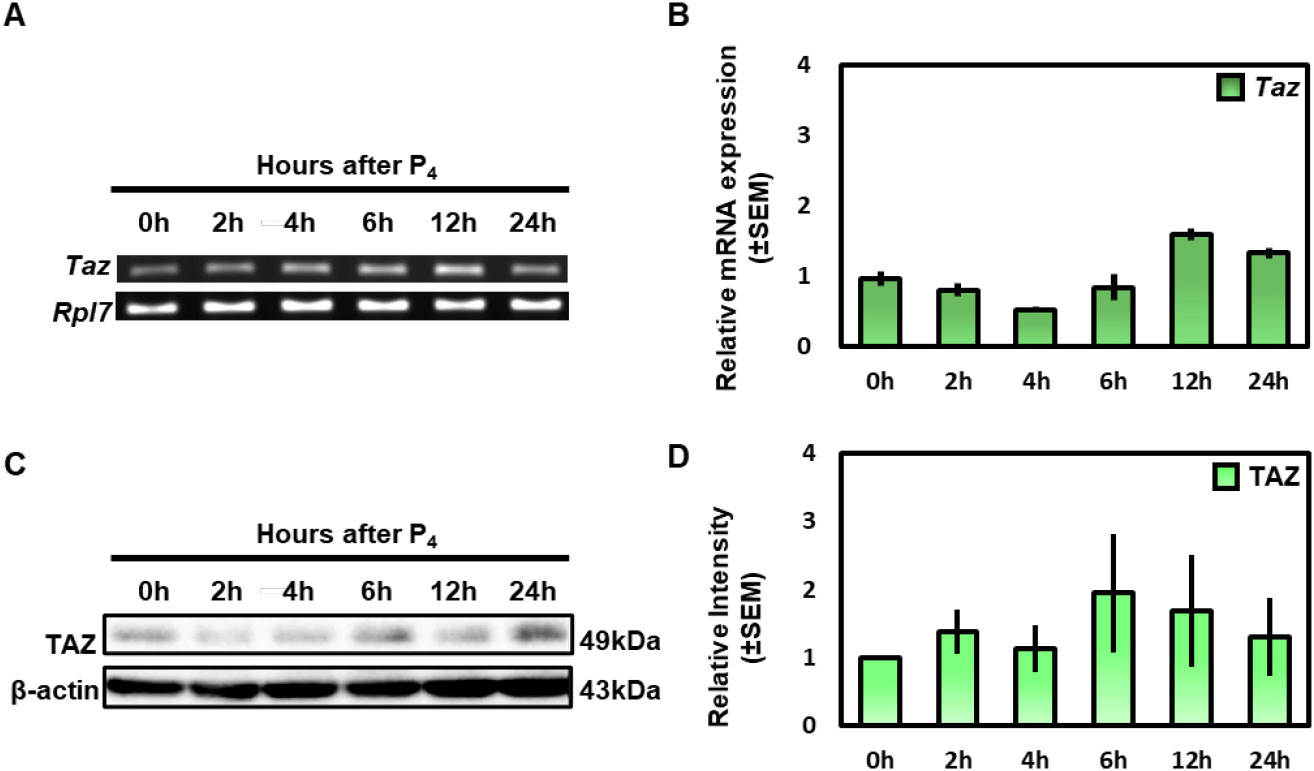
First, OVX mice were treated with progesterone, then the uteri were collected at 0, 2, 4, 6, 12 and 24 h later. To investigate the hormonal responsiveness to progesterone, mRNA expression of Amphiregulin (Areg) (Das et al., 1995), and Homeobox A10 (Hoxa10) (Taylor et al., 1998) which are known as progesterone-induced genes were investigated using the qRT-PCR (Supplementary Fig. 1B).
To investigate the Taz mRNA expression, we performed RT-PCR and qRT-PCR analysis. The results showed that the Taz mRNA expression did not change significantly over time after progesterone treatment (Fig. 3A and B). Furthermore, we performed western blot analysis to investigate the protein level of TAZ after progesterone treatment. Consistent with mRNA data, the expression of TAZ protein showed no difference according to progesterone treatment (Fig. 3C and D). These results suggest that TAZ expression is not affected by progesterone in the uterus.
In the next study, since estrogen is another important regulatory hormone in the uterus, the expression of TAZ is affected by estrogen being investigated. OVX mice were administered estrogen, and uteri were collected at 0, 2, 4, 6, 12, and 24 h. Hormone responsiveness to estrogen was referenced by evaluating mRNA levels of Cysteine-rich angiogenic inducer 61 (Cyr61) (Walmer et al., 1992; MacLaughlan et al., 2007), Ras Related Dexamethasone Induced 1 (Rasd1) (Kim et al., 2017), and Lactoferrin (Ltf) (Walmer et al., 1992) which are known as estrogen-induced genes (Supplementary Fig. 1A).
RT-PCR and qRT-PCR were analyzed to find out the effect of estrogen on the Taz mRNA level. The Taz expression was increased time-dependent manner after estrogen treatment, and peaks at 4 h (Fig. 4A and B). We also performed a western blot to investigate the protein level of TAZ. Expression of TAZ protein showed a dramatic increase at 6 h and 12 h compared to 0 h and dropped to 24 h (Fig. 4C and D).
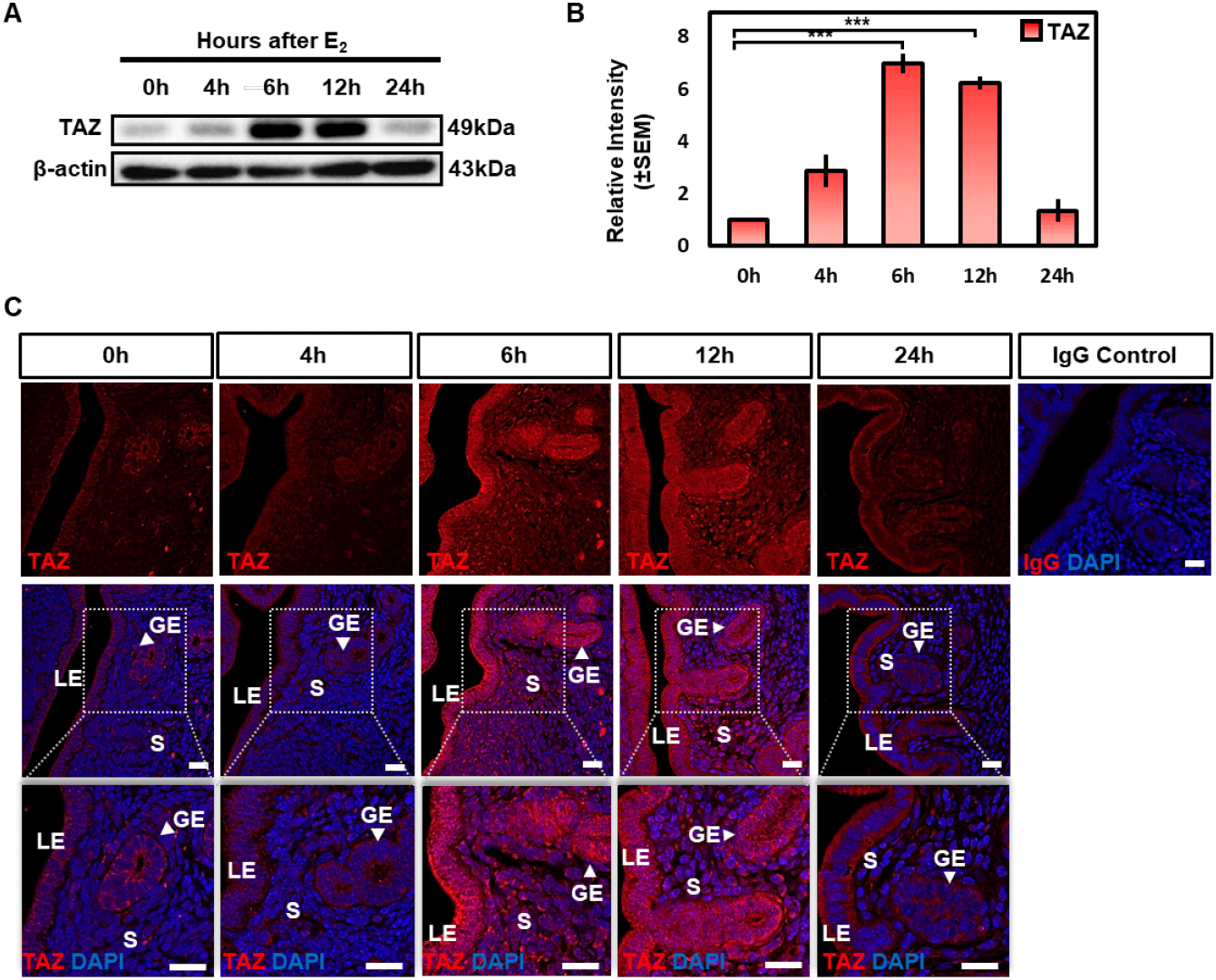
Interestingly, different from the normal uterus, TAZ expression was dominantly expressed in luminal and glandular epithelium and rarely expressed in stromal cells in OVX mouse uterus (Fig. 4E). After estrogen treatment, TAZ expression was induced from glandular and LE cells to stromal cells (Fig. 4E). Then TAZ expression was significantly decreased and restricted to luminal and glandular epithelium at 24 h as same as at 0 h (Fig. 4E). In addition, TAZ was predominantly expressed in the cytoplasm at 0 h to 4 h but was expressed both in the nucleus and cytoplasm at 6 h and 12 h (Fig. 4E). Collectively, these results imply that estrogen induces the expression level of TAZ and activates the transcriptional activity of TAZ in both glandular, LE cells and stromal cells.
To determine whether the estrogen-induced accumulation of TAZ protein is mediated via estrogen receptors-dependent, the OVX mice were pretreated with an estrogen receptor selective antagonist ICI 182,780 (ICI) 30 min before estrogen administration. The expression of TAZ protein was examined 6 h after estrogen treatment. Western blot analysis showed that estrogen-induced TAZ protein was significantly blocked by ICI treatment (Fig. 5A and B).
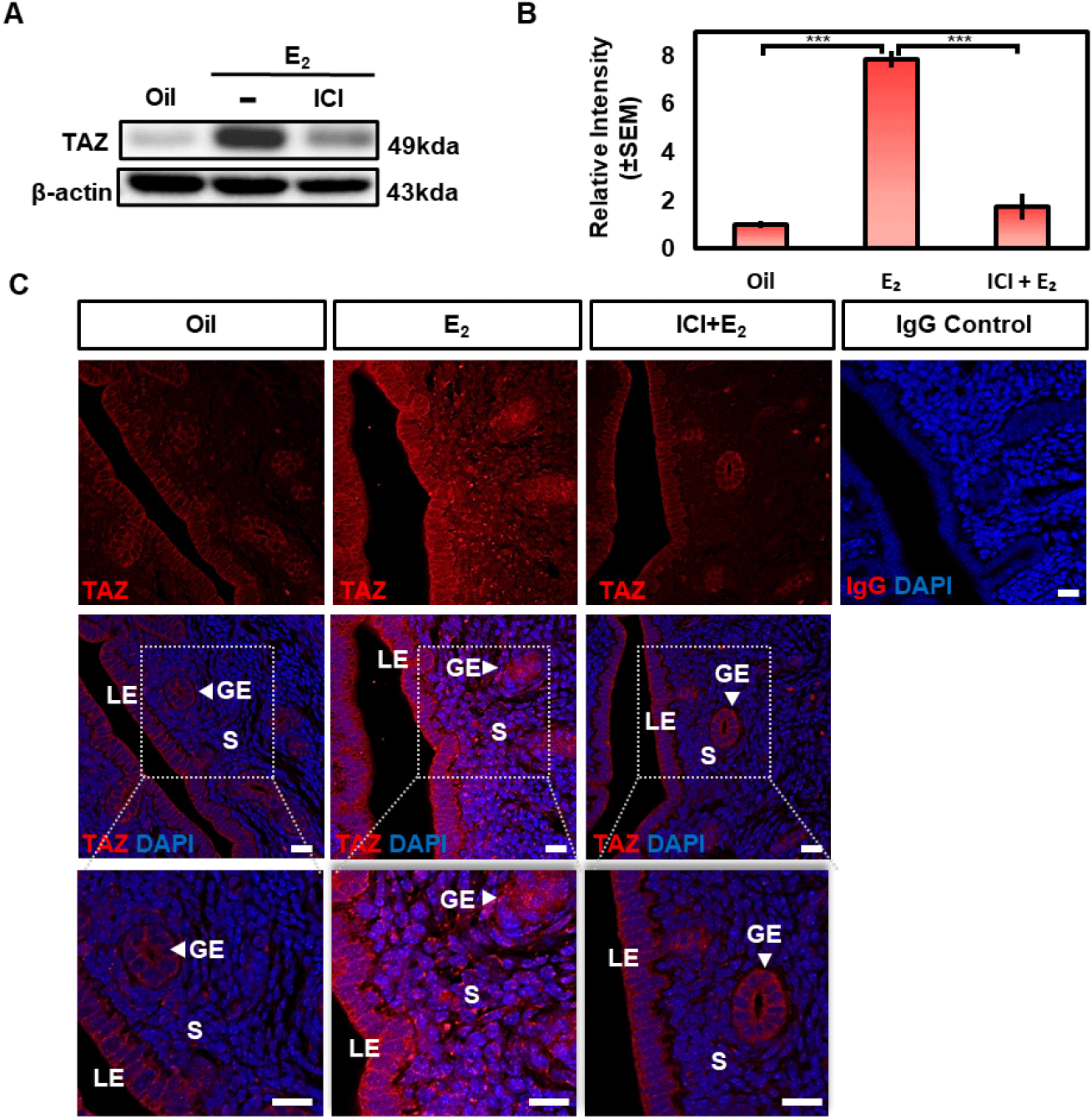
Also, immunofluorescence analysis showed that ICI treatment suppressed estrogen-induced TAZ expression and nuclear translocation in epithelial and stromal cells to a similar extent to that of oil treated uterus (Fig. 5C). Taken together, these results suggest that estrogen induces the expression of TAZ by estrogen receptor-mediated pathway in the epithelium and stromal cells of the uterus.
DISCUSSION
TAZ, which functions as a transcriptional coactivator was known to be regulated by nuclei-cytoplasmic localization in a phosphorylation-dependent manner under the Hippo signaling pathway (Lei et al., 2008). The Hippo signaling pathway which is well conserved in mammals was first discovered in drosophila (Badouel & McNeill, 2011). The Hippo signaling is known to play an anti-tumor function by limiting organ size via regulating various cellular processes such as cell proliferation, apoptosis, and differentiation (Kim & Jho, 2018).
Recently, several studies reported on the Hippo signaling factors in the endometrium. STK3/4 and YAP were regulated by estrogen during the estrous cycle in the mouse uterus (Moon et al., 2019, 2022). Moreover, several main downstream targets of YAP/TAZ such as Cyr61 (MacLaughlan et al., 2007), Ctgf (Maybin et al., 2012), Amot, Amotl1, and Amotl2 (Huang et al., 2018), Birc5 (Cho et al., 2020) were regulated by steroid hormones in the uterine tissue or endometrial cells. These suggest that the Hippo signaling factors act as an important signaling pathway in the endometrium that is dynamically regulated by ovarian steroid hormones.
Also, Chen et al. reported that YAP showed high expression in human decidual cells compared to ESCs and induces the decidualization of ESCs (Chen et al., 2017). Yu et al. suggested that YAP was essential for uterine decidualization in mouse uterine stromal cells (Yu et al., 2022). Also, TAZ expression was increased in pregnant mice uteri compared to non-pregnant mice and induced decidualization in mouse ESCs (Huang et al., 2018). These results imply that the normal regulation of the Hippo signaling pathway plays a role in dynamic uterine signaling mechanisms for a successful pregnancy.
TAZ activates target genes involved in various physiological cellular events such as cell proliferation, migration, apoptosis, differentiation, and senescence by interacting with TEADs transcriptional factors (Varelas et al., 2008; Di Palma et al., 2009; Zhang et al., 2009; Jeong et al., 2010; Wang et al., 2016; Kim et al., 2019). The uterus undergoes various cellular processes including cell proliferation, apoptosis, and differentiation during the estrous cycle (Evans et al., 1990). Collectively, TAZ, the main downstream factor of the Hippo signaling, may be an important signaling factor that contributes to the dynamic changes in the uterine endometrium. However, the studies on the regulation of TAZ by hormones in the uterus have not been elucidated. Therefore, this report investigated the expression pattern of TAZ during the estrous cycle in the mouse uterus and the effect of estrogen and progesterone on TAZ expression in the OVX mouse uterus.
In the present study, we showed that Taz mRNA is highly expressed in various mouse tissues, especially in the kidney, lung, and ovary (Fig. 1A). This data was consistent with a previous study (Yue et al., 2014). Interestingly, Taz expression was also high in the uterus compared to other tissues, suggesting that Taz may play a major role in the uterine endometrium.
Since this study focused on Taz expression in mouse uterus, we investigated the regulation of TAZ expression in the mouse uterus during the estrous cycle. Immunofluorescence analysis showed that the TAZ nuclear localization of both epithelial and stromal cells was predominantly observed in the estrus than in other stages (Fig. 2C). The mouse estrous cycle consists of four phases: proestrus, estrus, metestrus, and diestrus. The estrus stage is the period immediately after ovulation which is induced by a surge in luteinizing hormone due to a persistent increase in estrogen levels during the proestrus. Wood et al. reported that LE and stromal cell (p<0.05) proliferation rate reached the maximum level in the estrus (Wood et al., 2007). Also, they demonstrated that the apoptosis rate of luminal, glandular epithelial cells, and stromal cells were lowest at the estrus stage (Wood et al., 2007). The transcriptional activity of TAZ is known to be regulated by nuclear-cytoplasmic translocation. Upon entering the nucleus, TAZ functions as a coactivator that promotes gene targets by interacting with TEADs (Mahoney et al., 2005; Zhang et al., 2009). TAZ overexpression was known to promote cell proliferation and epithelial-mesenchymal transition in breast epithelial cell lines (Lei et al., 2008). Also, Yu et al. demonstrated that TAZ overexpression induced proliferation and prevented apoptosis in mouse ESCs (Yu et al., 2021). Collectively, these results suggest that TAZ may play a role in promoting cell proliferation and inhibiting apoptosis, particularly LE and stromal cells through nuclear-cytoplasmic translocation regulated by fluctuations of ovarian hormones, estrogen, and progesterone at the estrus.
Next, we investigated the effect of steroid hormones on TAZ expression using the OVX mouse model as in previous studies (Kim et al., 2017; Cho et al., 2020). Estrogen induced both mRNA and protein levels of TAZ time-dependently in the OVX mouse uterus (Fig. 4). Taz mRNA was highest in 4 h and was higher than 0 h uteri up to 24 h (Fig. 4A and B), whereas protein showed a statistically significant increase from 6 h to 12 h and then showed a marked decrease at 24 h after estrogen treatment (Fig. 4C and D). These results, showing different increase patterns between mRNA and protein, suggest that estrogen may be involved not only in the transcriptional induction of the Taz gene but also in the post-translational modification of the TAZ protein via several non-genomic pathways. Consistent with the western blot result, immunofluorescence showed that expression level and nuclear translocation of TAZ were increased after estrogen treatment in a time-dependent manner (Fig 4E). Finally, estrogen-induced TAZ protein was efficiently decreased after pretreatment of estrogen receptor antagonist ICI 182,780 (Fig. 5). Several studies have reported that estrogen stimulates the proliferation of uterine epithelial cells in OVX mice, and high expression of TAZ was usually involved in inducing cell proliferation (Quarmby & Korach, 1984; Lei et al., 2008; Zhang et al., 2009). Collectively, these suggest that estrogen induces TAZ expression and activates its transcriptional activity to induce epithelial cell proliferation through estrogen receptors in the uterine endometrium.
Unlike the normal uterus (Fig. 2C), TAZ was dominantly located in luminal and glandular epithelium and rarely in stromal cells in the OVX mouse uterus (Fig. 4E). Then TAZ expression was increased from glandular and LE cells to stromal cells after estrogen treatment (Fig. 4E). This implies that estrogen may play an important role in inducing TAZ expression in stromal cells.
Interestingly, TAZ expression was increased in the uterus of OVX mice (Fig. 4), but there was no significant change in the total amount of mRNA or protein of TAZ according to the estrous cycle (Fig. 2). These results might be due to technical problems, but several reasons can be present. The first reason is that the normal uterine endometrium undergoes a dynamic remodeling controlled by the synergetic effects of estrogen and progesterone during the estrous cycle (Evans et al., 1990; Dharma et al., 2001; Wood et al., 2007; Bertolin & Murphy, 2014). However, such synergetic effects are almost excluded in the uterus of the OVX mouse model. The present study showed that the TAZ expression of OVX mouse uterus was not significantly changed in both mRNA and protein levels after progesterone treatment (Fig. 3). These results imply that progesterone alone may not significantly affect the TAZ expression. However, Yu et al. reported that TAZ showed high expression in pregnant mouse uterus compared to non-pregnant mouse and played an essential role in decidualization of mouse ESCs (Yu et al., 2021). Decidualization occurs through a series of regulations of estrogen and progesterone for successful implantation. So, there may be a possibility that there is a synergetic effect of estrogen and progesterone on TAZ expression. The second reason is, since there is a difference between the serum estrogen level in a normal mouse and the OVX model experimentally administered with estrogen (200 ng/mouse), there may be differences in estrogen-mediated changes in TAZ expression.
However, we need further studies for a better understanding of the regulatory mechanisms between estrogen and TAZ expression in the mouse uterus. First, it is necessary to examine the change of phosphorylated TAZ (pTAZ) levels by estrogen in the uterus. The transcriptional activity of TAZ was known to be regulated by the phosphorylation of several regions by LATS1/2 kinases. When Ser89 of TAZ is phosphorylated by LATS1/2, cytoplasmic sequestration of TAZ occurs by interacting with 14-3-3 protein (Lei et al., 2008). On the other hand, if LATS1/2 phosphorylate Ser311 of TAZ, Casein kinase 1 (Ck1) subsequently phosphorylates Ser314, leading to its polyubiquitylation and proteasomal degradation by the SCF/CRL1 (β-TrCP) E3 ligase (Liu et al., 2010). Therefore, the mechanisms regulating nuclei-cytoplasmic migration and protein stability of TAZ are important factors in increasing its protein levels so the need for further studies on the expression of pTAZ by estrogen is raised. Second, it is necessary to investigate which non-genomic pathways of estrogen receptor (ER) contributed to inducing TAZ expression. The increase of TAZ expression occurring at 6 h is usually considered to be the early response by estrogen (Groothuis et al., 2007). Estrogen can activate signal-transduction mechanisms through membrane-bound ER or GPER that result in fast estrogen-induced biological responses (Lösel & Wehling, 2003). Extra-nuclear ER signaling pathways involve the activation of mitogen-activated protein kinase (MAPK) pathways and protein kinase B (known as AKT). Several studies reported the regulation of TAZ expression through these signaling cascades by several RTKs under the Hippo signaling (Azad et al., 2018; van Soldt & Cardoso, 2020). Also, Zhou et al. reported that estrogen activated TAZ by increasing nuclear localization by inhibiting LATS1/2 activity of phosphorylation on Ser89 in breast cancer cells (Zhou et al., 2015). These suggest the possibility that estrogen may induce TAZ expression may be caused by several non-genomic signaling pathways of E2 in the uterine endometrium.
Finally, several studies also reported on the association between the main downstream of Hippo signaling, YAP/TAZ, and endometrial diseases. High expression of YAP was associated with endometriosis in several studies (Pei et al., 2019). Romero-Pérez et al. observed that high TAZ expression correlated with the most aggressive endometrial cancer, and its overexpression promoted tumorigenicity, such as mobility and invasiveness of endometrial cancer cell lines (Romero-Pérez et al., 2015). Also, Zhan et al. reported that TAZ was predominantly expressed in the nucleus of the endometrioid adenocarcinoma tissue (Zhan et al., 2016). These imply that abnormal expressions of YAP/TAZ may result in endometrial diseases. Disruption of the tightly regulated balance of estrogen and progesterone can potentially lead to uterine diseases (Azad et al., 2018). Therefore, it is considered that the study on the correlation between TAZ regulation and uterine hormones may be important research in the therapeutic study of uterine diseases.
In conclusion, we demonstrated that TAZ transcriptional activity is dynamically regulated through the estrous cycle and is induced and activated by estrogen via estrogen receptors in the endometrium. Although more studies are needed, our findings will provide a better understanding of the relationship between the dynamically regulated uterine environment and the Hippo signaling pathway, further providing good clues for studying the mechanisms of endometrial diseases.

