INTRODUCTION
In the mammalian ovary, sex steroid hormones that include 17β-estradiol (E2) and progesterone (P4) are mainly produced in follicles as well as corpus luteum (CL) (Adashi, 1994). To produce E2, androgens that include androstenedione (A4) and testosterone (T) are first synthesized in thecal cells, followed by conversion to E2 by aromatase in granulosa cells of the follicles. Androgen production in theca cells and E2 production in granulosa cells are stimulated by luteinizing hormone (LH) and follicle-stimulating hormone (FSH), respectively (Miller and Auchus, 2011). In contrast to E2 production in follicles, a relatively higher concentration of P4 is produced in luteal cells of CL (particularly in active or newly formed CL). In response to LH, this could be readily achieved in CL because luteal cells lack cytochrome P450 17β-hydroxylase (CYP17) that catalyzes the conversion of P4 to A4 (Devoto et al., 2002). Ultimately, P4 secreted from CL is pivotal in regulating and maintaining uterine functions and integrity.
In both theca cells and luteal cells, an initial stage of the steroidogenic process involved with cholesterol intake and translocation and subsequent pregnenolone synthesis is commonly achieved in mitochondria. Once steroidogenesis is stimulated, hydrophobic and cellular cholesterol residing in the cytoplasm are transported to the outer mitochondrial membrane by binding to carrier proteins, such as sterol carrier protein 2 (SCP2). The steroidogenic acute regulatory (StAR) protein translocates the cholesterol to the inner mitochondrial membrane, which converts cholesterol to pregnenolone by cytochrome P450 side-chain cleavage (P450scc; CYP11A1). In the case of luteal cells, P4 is finally synthesized from pregnenolone by activating 3β-hydroxysteroid dehydrogenase (3β-HSD; HSD3B2) and secreted into the bloodstream (Stocco et al., 2007). Therefore, mitochondria are an essential cellular organelle in steroidogenesis (Miller, 2011).
Recently, the specific engagement of the concept regarding mitochondrial shaping and dynamics with early stages of steroidogenesis was suggested in testicular Leydig cells as well as in ovarian luteal cells (Duarte et al., 2012; Park et al., 2019; Plewes et al., 2020). Among the proteins associated with mitochondrial dynamics, GTPase dynamin-related protein 1 (Drp1) has been particularly accentuated in steroidogenic machineries (Park et al., 2019; Plewes et al., 2020). Although the specific involvement of Drp1 with steroidogenesis has been demonstrated in luteal cells of bovine CL in vitro (Plewes et al., 2020), its actual relationship with ovarian steroidogenesis during the estrous cycle remains unknown. Thus, in the present study, Drp1 proteins were localized, and their expression levels were monitored in the ovaries during each stage of the estrous cycle in rats. The purpose of this study was to determine whether Drp1 proteins in luteal cells of the CL correlate with changes in blood P4 levels in each stage of the estrous cycle of rats.
MATERIALS AND METHODS
Hematoxylin and neutral buffered formalin (NBF) were purchased from Sigma Chemical (St. Louis, MO, USA). Complete Protease Inhibitor Cocktail Tablet was from Roche Applied Science (Mannheim, Germany). Progesterone enzyme-linked immunosorbent assay (ELISA) kits were purchased from IBL (Hamburg, Germany). The western enhanced chemiluminescence (ECL) detection reagent was purchased from Bio-Rad (Hercules, CA, USA). Anti-3β-HSD, Mfn1, Mfn2, Actin antibodies were obtained from Santa Cruz Biotech (Santa Cruz, CA, USA). Anti-pDrp1 (Ser 637) antibody, rabbit and mouse IgG-conjugated with horseradish peroxidase were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-Fis1 antibody was from Alexis Biochemicals (Lausen, Switzerland). Anti-Drp1 and Opa1 antibodies were obtained from BD Transduction Laboratories (Lexington, KY, USA). VECTASTAIN® Elite ABC Kit, VECTASHIELD® Mounting Medium were from Vector Laboratories (Burlingame, CA, USA).
Adult female Sprague-Dawley rats (12-week-old) were purchased from SamTako Bio-Korea (Osan, Korea). The rats were housed in a climate-controlled (21±2°C) animal room at a constant 12-h light and 12-h dark cycle, with unlimited access to rat chow. All procedures were performed in accordance with protocols approved by the Dong-A University Animal Care and Use Committee. Vaginal smears were prepared from each animal daily between 8:00 am and 10:00 am by lavage with 0.9% saline and the fluid was spotted thinly on a microscope slide. The dried slides were stained with 0.1% trypan blue in deionized water and were allowed to dry. The determination of the estrus cycle stage was based on microscopic examination, as described (Westwood, 2008). At each stage of the estrous cycle, rats were sacrificed by carbon dioxide asphyxiation and blood samples were collected using the techniques of cardiac puncture. The right ovaries were removed and fixed in NBF for histological examination. The left ovaries were placed in cold phosphate buffer solution (PBS) for further biochemical analysis.
P4 serum concentrations were determined in duplicate samples using P4 ELISA kits (IBL, Hamburg, Germany) according to manufacturer’s instructions. The sensitivity of the P4 assay was 0.045 ng/mL, and the intraassay and interassay coefficients of variation (CVs) were 6.4% and 6.6%, respectively.
To obtain residual ovaries (retaining theca-interstitial cells), granulosa cells and oocytes were mostly removed by follicular puncture as described previously (Rao et al., 1991). Briefly, granulosa cells from ovaries were collected in ice-cold M199 medium by follicle puncture with a 27-gauge hypodermic needle and discarded. The residual ovarieswere thoroughly washed with M199 to release undissociated granulosa cells, transferred into clean tubes, frozen on dry ice, and stored at −80°C.
Residual ovaries were homogenated and lysed in lysis buffer [300 mM NaCl, 0.5% Triton X-100, 50 mM Tris-HCl (pH 7.4), 25 mM NaF, 1 mM Na3VO4, 10 mM Na4P2O7, and protease inhibitor] for 30 min on ice. The lysates were centrifuged at 13,000×g at 4°C for 20min, the supernatants were collected and protein concentration was measured using the BCA protein assay kit. 25 micrograms of protein extract with sodium dodecyl sulfate (SDS)-loading buffer [2% SDS, 100 mM DTT, 10% glycerol, 0.02% bromophenol blue, and 50 mM Tris-HCl, pH 6.8] was electrophoretically separated on a 8%-15% gradient SDS-PAGE gel, and transferred onto a nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk dissolved in Tris-buffered saline (TBS) buffer containing 0.05% Tween-20 at RT for 1 h. The blots were incubated with primary antibodies, followed by incubation with appropriate HRP-conjugated secondary antibodies. The signals were detected with ECL detection reagent in the LAS-4000 (Fuji, Tokyo, Japan). Actin was used as internal control for total cellular proteins.
For immunohistochemical staining, deparaffinized and hydrated ovary sections were treated in 3% H2O2 for 5 min and rinsed with PBS for 15 min. Subsequently, the Vectastain ABC kit (Vector Lab., Burlingame, CA, USA) was used, according to manufacturer’s instructions. The nuclei were counterstained with hematoxylin. For negative controls, rabbit IgG (1 mg/mL) instead of the primary antibodies was added to the reaction. The results were observed using a ScanScope digital slide scanning system (Aperio Technologies, Vista, CA, USA) at the Neuroscience Translational Research Solution Center (Busan, Korea).
RESULTS AND DISCUSSION
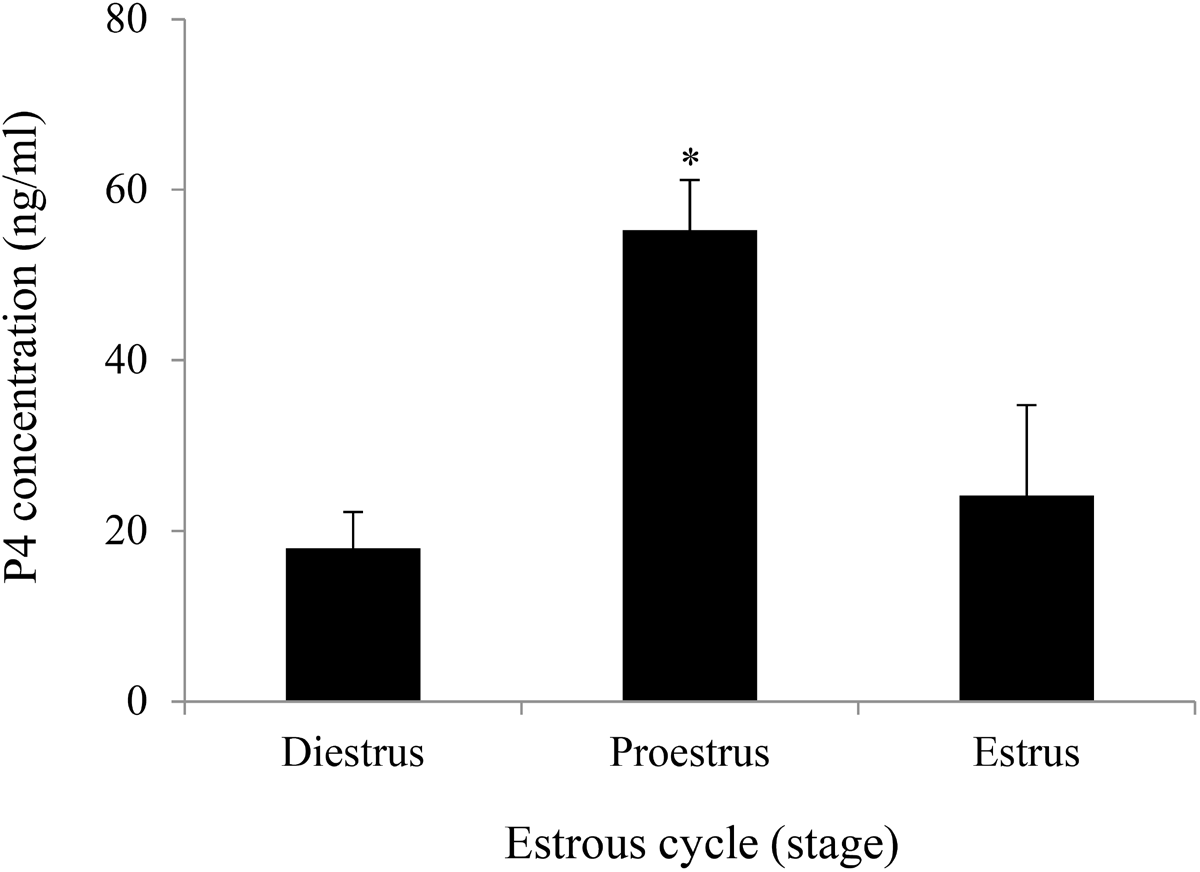
Similar to the human menstrual cycle, serum E2 and P4 levels in rodents are periodically altered in response to FSH and LH during the estrous cycle. In humans, E2 and P4 levels are the highest in the late follicular and midluteal phases, respectively. In contrast, both E2 and P4 levels generally show a sharp elevation during the proestrus phase in rodents. In particular, intensive P4 synthesis occurs in luteal cells of the newly formed CL after ovulation. Therefore, considering the amount of sex steroid hormones produced in the ovary, steroidogenic activity can mostly be high in luteal cells of the active CL at proestrus. In the present study, serum P4 levels were initially monitored during the estrous cycle in the rat. The results confirmed that P4 levels were remarkably higher at proestrus than at estrus or diestrus (Fig. 1).
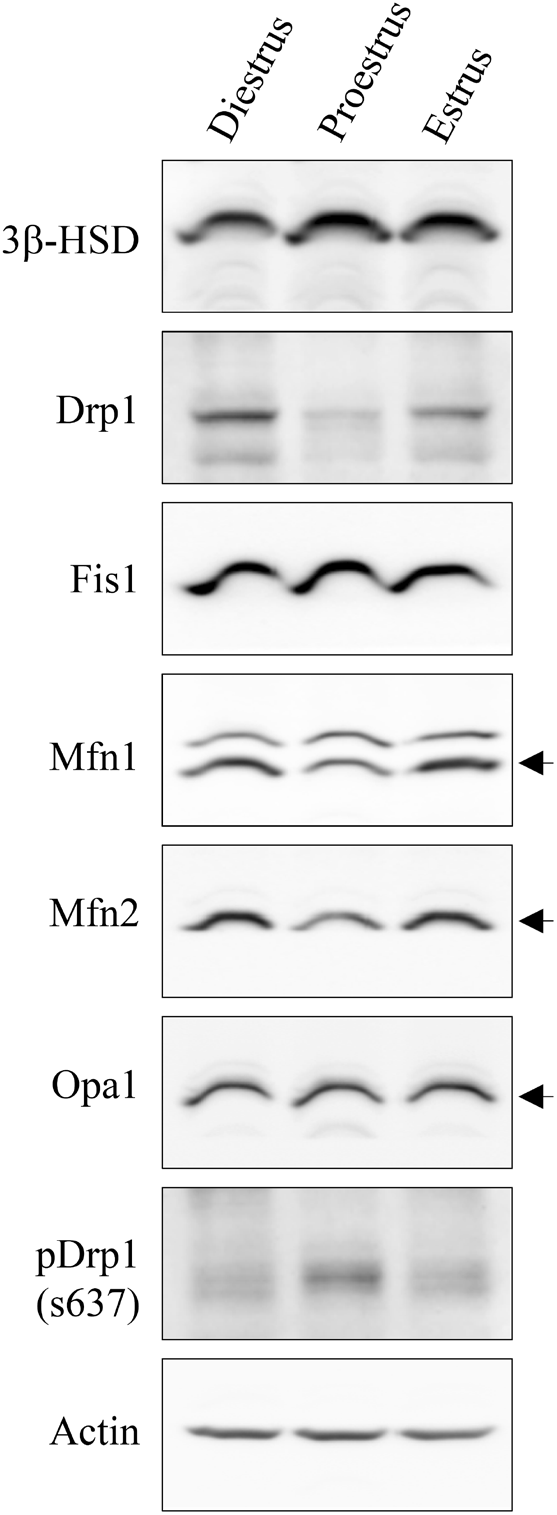
In cells, the mitochondria contribute to energy conversion, metabolism, signaling, aging, cancer, apoptosis, and steroidogenesis. In the cytoplasm, mitochondria often change shape due to fusion and fission processes, called mitochondrial dynamics (Bereiter-Hahn and Vöth, 1994). Unbalanced fission causes mitochondrial fragmentation and more extensive fusion results in mitochondrial elongation (Chen et al., 2003). Although several proteins associated with mitochondrial fission and fusion have been identified so far, Drp1 and Fis1 are representative of fission (Smirnova et al., 2001; James et al., 2003) and Mfn1, Mfn2, and Opa1 of fusion (Ishihara et al., 2004; Olichon et al., 2002). The phosphorylation of Drp1 at Ser 637 suppresses Drp1 translocation to the mitochondria and thus inhibits fission (Chang & Blackstone, 2007). In this study, changes in the protein levels of Drp1, phospho-Drp1 (s637), Fis1, Mfn1, Mfn2, Opa1, and 3β-HSD in ovarian tissue (residual ovaries) were examined during the estrous cycle using western blotting (Fig. 2). While Fis1 and Opa1 protein levels did not show significant changes during the estrous cycle, Drp1, Mfn1, and Mfn2 proteins exhibited relatively lower levels at proestrus than at estrus or diestrus (Fig. 2). Based on these results, it is assumed that mitochondrial fission and/or fusion in ovarian cells may occur more actively during estrus and diestrus than during proestrus. However, 3β-HSD, a representative enzyme for steroidogenic activity, particularly in luteal cells, showed higher levels at proestrus than at estrus or diestrus (Fig. 2). Together, the degree of mitochondrial dynamics-related protein expression exhibited an inverse relationship with steroidogenic activity and production in ovarian cells. Recently, it was demonstrated that phosphorylation of Drp1 at serine 637 is crucial for steroidogenesis in Leydig cells (Park et al., 2019) as well as in luteal cells (Plewes et al., 2020). Therefore, we examined the possibility of association of Drp1 phosphorylation (s637) with P4 production in the ovary. Western blot analysis distinctly showed that Drp1 phosphorylation (s637) was higher in proestrus than in estrus or diestrus (Fig. 2). This indicates that the extent of Drp1 phosphorylation (s637) correlates with P4 production levels in the ovary.
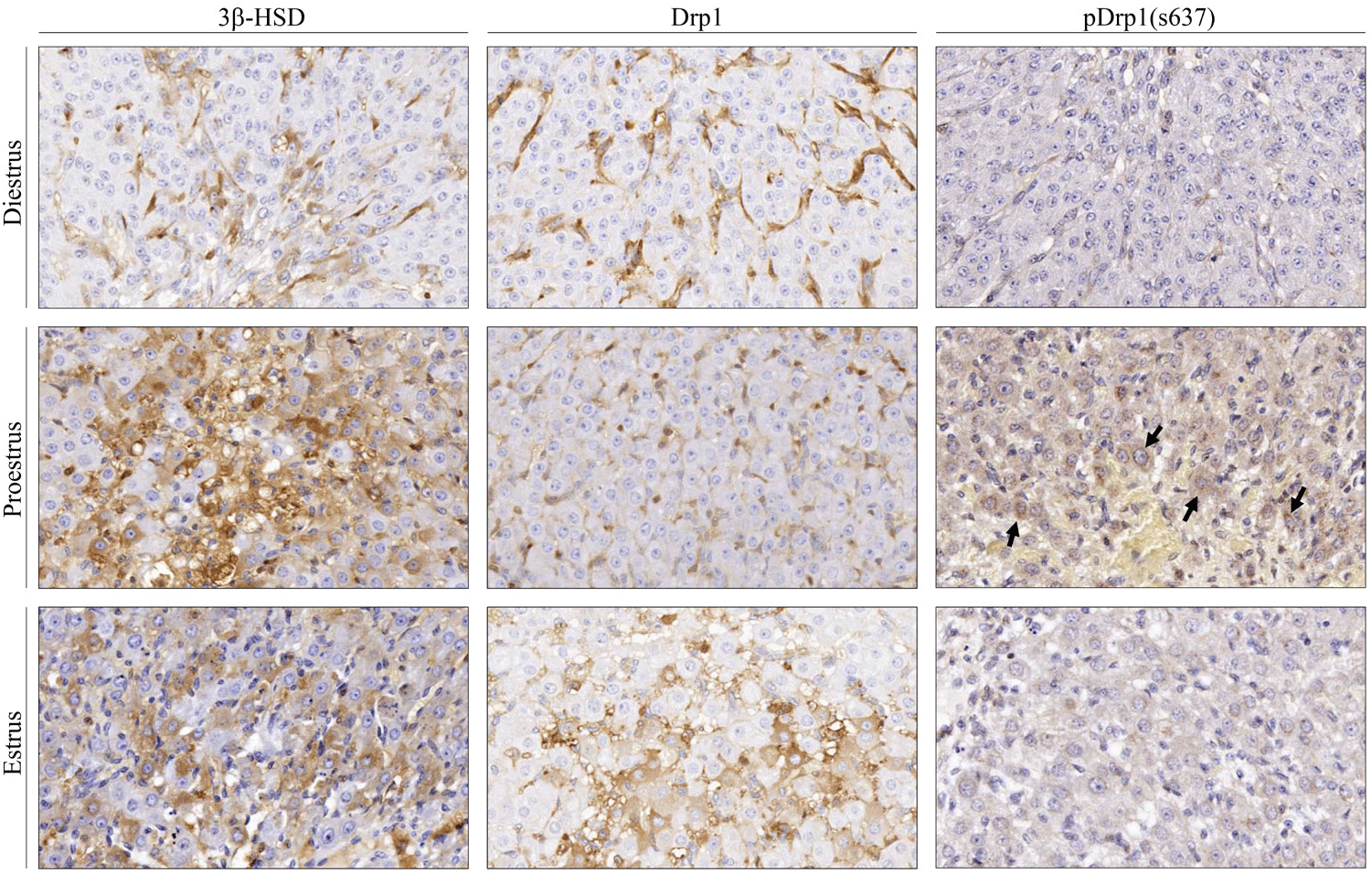
Next, Drp1, pDrp1(S637), and 3β-HSD were immunohistochemically localized in the rat ovaries at each stage of the estrous cycle. In this study, microscopic observations and analyses were restricted to the CL. As seen in Fig. 3, immune-positive cells for Drp1, pDrp1 (s637), and 3β-HSD were all localized in the cytoplasm of luteal cells in the CL. The immune-positive cells for 3β-HSD were more frequently seen in the CL at proestrus than at estrus or diestrus, and the immunoreactivity was also more intense in luteal cells at proestrus than at other estrous stages. Immunoreactivity for Drp1 in luteal cells at proestrus was weaker than that at estrus or diestrus. However, pDrp1 (s637) immune-positive cells were mostly detected in luteal cells at proestrus. In luteal cells at estrus and diestrus stages, immune-positive cells for pDrp1 (s637) were rarely observed in the CL throughout the ovaries. These results imply that steroidogenesis (P4 production) in the CL is closely related to phosphorylation of Drp1 at serine 637.
Taken together, this study presents evidence that Drp1 phosphorylation at serine 637 is an important step in steroidogenesis in the CL. We believe that mitochondrial fission and/or fusion (mitochondrial dynamics) are closely associated with steroidogenesis in luteal cells as well as in theca/granulosa cells in the ovary. Further studies on the regulatory mechanism(s) by which mitochondrial fission and fusion are balanced in luteal cells will help understand the optimal cellular environment and conditions for steroidogenesis.

