INTRODUCTION
Aplysia kurodai (Gunso in Korean) belongs to the family Aplysiidae, order Anaspidea, subclass Ophithobranchia, class Gastropoda, and is a major species in Korea. The genus Aplysia has 50 species that are distributed worldwide (Beeman, 1968; Klussmann-Kolb, 2004). Since many Aplysia species have a relatively simple nervous system with large neurons, they have been of a major interest to neurobiologists and physiologists (Kandel 1979; Kaang et al., 1993). Almost all opisthobranchs such as Cephalaspidea, Anaspidea and Nudibranchia, etc., are simultaneous hermaphrodites and have a functionally and structurally complex reproductive system (Hadfield & Switzer-Dunlap, 1984; Painter et al., 1985; Berry et al., 1992; Kress & Schmekel, 1992; Klussmann-Kolb, 2004). Most of opisthobranchs are capable of internal cross-fertilization, and perform both sex roles. The role of the male is the production and transference of autosperm (own sperm), and the role of the female is the storage of allosperm (sperm of another animal) and production of egg masses (Beeman, 1970; Hadfield & Switzer- Dunlap, 1984; Lee et al., 2014). Thus, opisthobranchs perform both function of male and female during copulation.
In order to understand how the sexual selection processes operate in a certain animal, it is essential to know the function of its reproductive system. The reproductive system of opisthobranchs consists of ovotestis, small hermaphroditic duct, accessory genital masses, large hermaphroditic duct, seminal receptacle, spermatheca, genital aperture, and genital groove (Beeman, 1970). The arrangement of the reproductive system, i.e., the pallial gonoduct from the post-ampullar to the common genital aperture varies greatly among species. According to the division of this duct, the reproductive system may be termed as monaulic, diaulic, or triaulic (Ghiselin, 1966). Many literatures are described on the opisthobranch reproductive system (Gosliner, 1981; Schrödl, 2000), that is based mainly on the anatomical and taxonomic viewpoints but functional analyses are comparatively lacking. The understanding of reproductive system provides systematic and phylogenetic information of opisthobranchs (Wägele, 2000; Dacosta et al., 2007; Ruthensteiner et al., 2007).
Like other opisthobranchs, Aplysia spp. have a complex reproductive system, and in particular, an interest because of an incompletely divided large hermaphroditic duct. Although the morphological feature and anatomy of Aplysia spp. have been studied previously, studies regarding the structure and function of reproductive system are comparatively lacking. This study investigated structure and function of the reproductive system in A. kurodai by means of anatomical, histological, and histochemical observation.
MATERIALS AND METHODS
A. kurodai were sampled by scuba diving at a depth of 3–5 meters in the coastal waters of Hamdeok, northeast of Jeju Island, Korea. Samples were collected from March to June during spawning season. The anatomical investigations were performed on live specimens which are about 200 g body weights. For marcroscopic investigation, the specimens were anaesthetized using 10% MgCl2 (Sigma Chemical Co., USA). The anatomy of A. kurodai was observed using a stereomicroscope and sketch morphological feature of reproductive system.
For histological and histochemical analysis on the reproductive system, the specimens were anaesthetized in 10% MgCl2, and each of the reproductive organs was dissected out of the animal. The pieces of the organs were fixed using 10% formaldehyde or Bouin’s solution, embedded in paraffin, sectioned into 5–6 μm, and stained with Hansen’s haematoxylin and 0.5% eosin. The ovotestis was stained with Azan. The sections of accessory genital mass were stained with Toluidine blue and additional sections were also stained with standard histochemical stains such as periodic acid Schiff and Alcian Blue.
The terminology for the different part of the reproductive system of opisthobranchs is very inconsistent throughout the literature. Therefore, the terminology used in this study mainly based on those of published by Hadfield and Switzer- Dunlap (1984), Painter et al. (1985), Gosliner (1994) and Klussmann-Kolb (2001a, b).
RESULTS
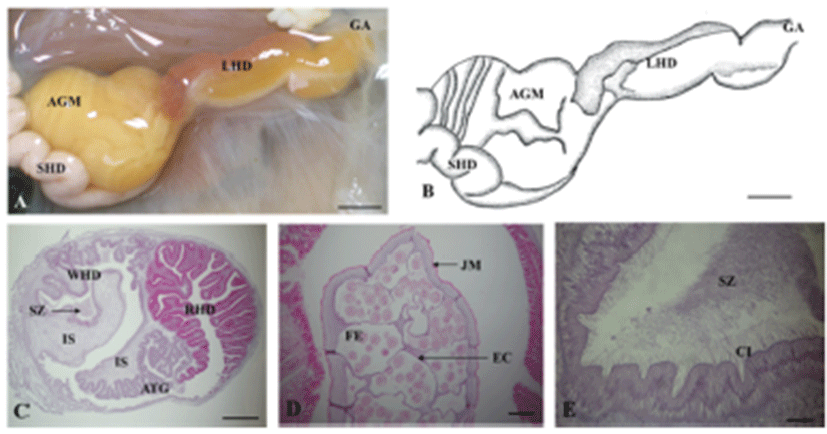
A. kurodai is bilateral symmetry, is covered with an irregular scattering of whitish spots. The sensory organs, such as the tentacles and eyes are located at head. The tentacles have 2 types: the cephalic tentacles in the form a fold of the body wall and conical rhiophores projecting dorsally from the surface of the neck. The long genital groove is located on the right dorsal side of the head. The mantle and mantle cavity are located between the two symmetrical parapodia that are separated posteriorly. The mantle forms the dorsal body wall of the visceral mass, and the shell is embedded in it. Under the shell is a single, large, folded gill. The siphon is folded to form a tubular structure posterior to the gill, with the anus in its center (Fig. 1A). The major portion of body cavity of A. kurodai is occupied by the digestive tract that can be divided into a foregut, midgut and a hindgut. The foregut consisted of a buccal mass, two salivary glands, and the esophagus. The midgut consisted of a large crop, grinding plates, and a true stomach (digestive pouch). The hindgut is made up of an intestine and a rectum (Fig. 1B). The buccal mass is large, dark red, and posterior to the buccal mass cerebral ganglia and other ganglia exist on top esophagus (Fig. 1C). The thin-walled crop that is contact with the esophagus is gray-brown in color and very large. It acts as a storage space and is filled with algae. The crop leads to the anterior gizzard (triturative stomach), which is composed of red muscle fibers, and its inner wall is lined with large pyramidal teeth. The posterior gizzard enters in the true stomach, i.e., a small digestive pouch. The stomach leads to a thin-walled, gray-brown intestine embedding into a large gray-brown digestive gland (Fig. 1D). The intestine makes several coils through the digestive gland and then contract into the anus (Fig. 1E).
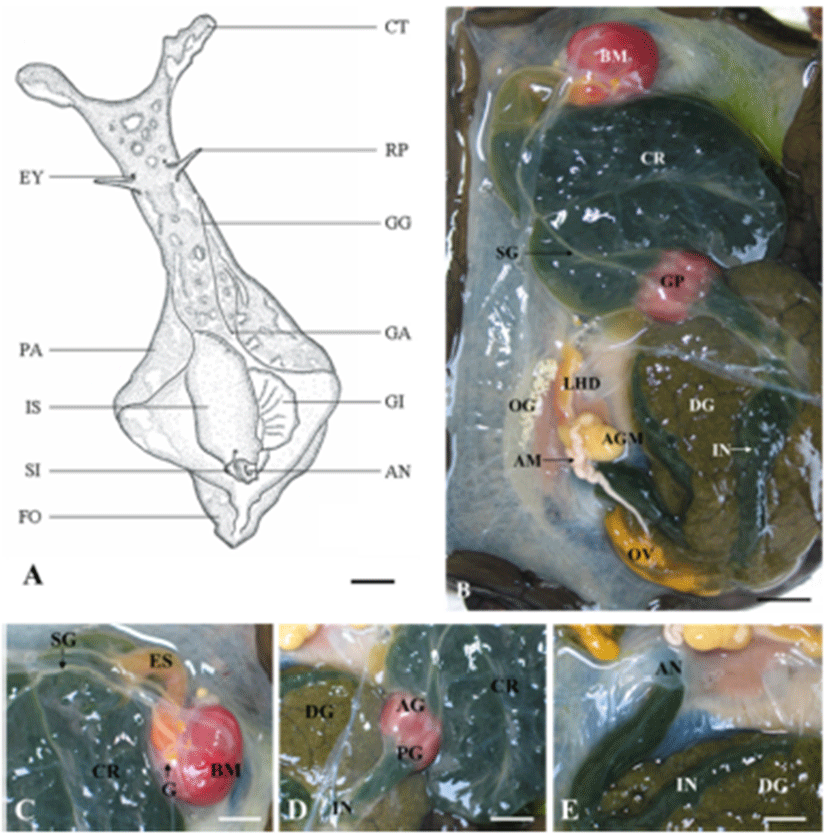
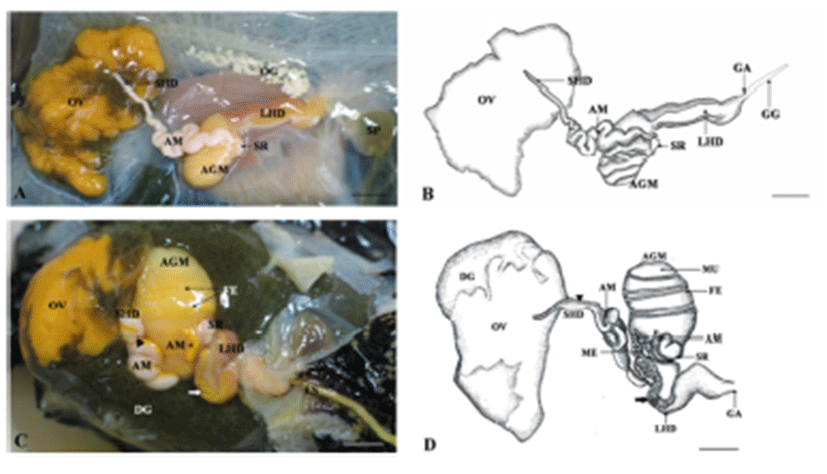
The reproductive system of A. kurodai is composed of ovotestis, small hermaphroditic duct, ampulla, accessory genital mass and large hermaphroditic duct (Fig. 2A, B). Following ovulation, eggs are transported from a posteriorly located ovotestis through the small hermaphroditic duct to the accessory genital mass. The accessory genital mass is connected to the large hermaphroditic duct. The large hermaphroditic duct opens externally as the common genital aperture. The external genital groove runs forward along the right external body surface from the common genital aperture to the penis, ensheathed in the right side of the head. The egg masses are released from genital aperture and moves along the external genital groove to be deposited on the substrate (Fig. 2C, D).
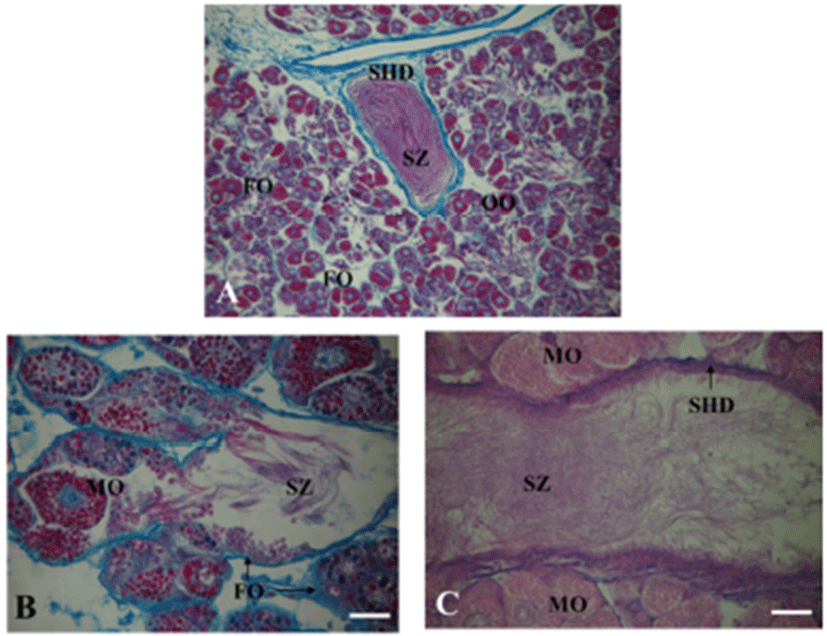
A. kurodai have a single unpaired gonad, the ovotestis with orange-yellow in color. It is generally embedded in the brownish digestive gland (Fig. 3). The ovotestis is composed of a large number of follicles, opens into a division of the small hermaphroditic duct (Fig. 3A). The oocytes and spermatozoa are developed in the same follicles, they gather into a division of the small hermaphroditic duct (Fig. 3B, C).
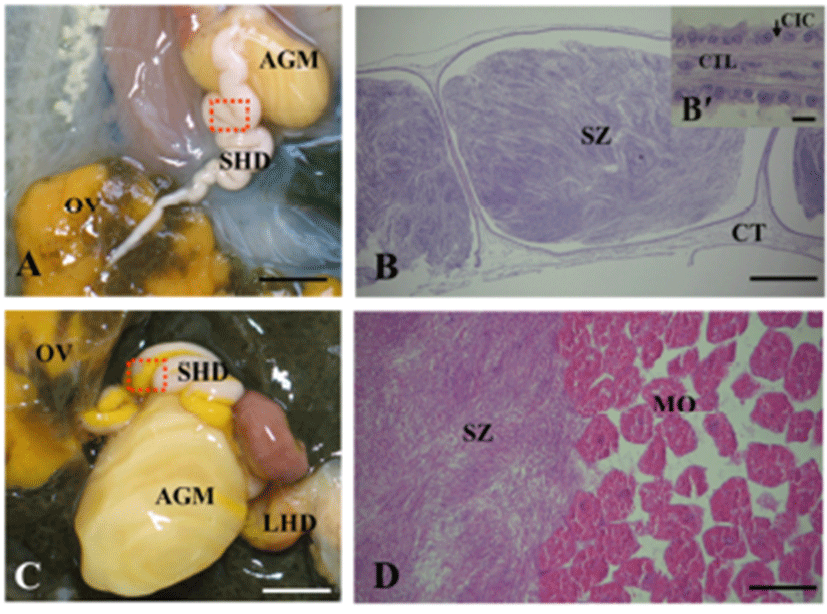
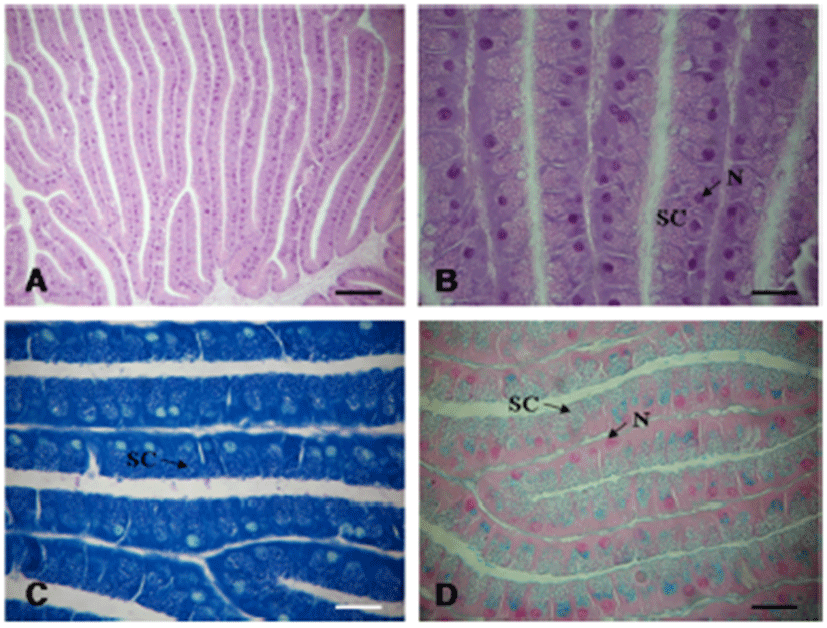
The small hermaphroditic duct is relatively straight and narrow at its origin from the ovotestis, but becomes progressively wider and more tortuous as it approaches in the accessory genital mass (Fig. 4A). The wide part of small hermaphroditic duct is called as the ampulla, and function as a storage organ for endogenous sperm (autosperm; the animal’s own sperm) and an oviduct (Fig. 4B to D). The wall of ampulla consists of a narrow epithelium with long ciliary tracts and is surrounded by muscular and connective tissue layers (Fig. 4B’).
The accessory genital mass is a large hemispherical organ located on the floor of the hemocoelom and is orangeyellow in color (Fig. 5). The accessory genital mass consists of three glands: albumen, membrane (winding) and mucus gland. This mass is connected to both the small and large hermaphroditic duct (Fig. 5A to D).
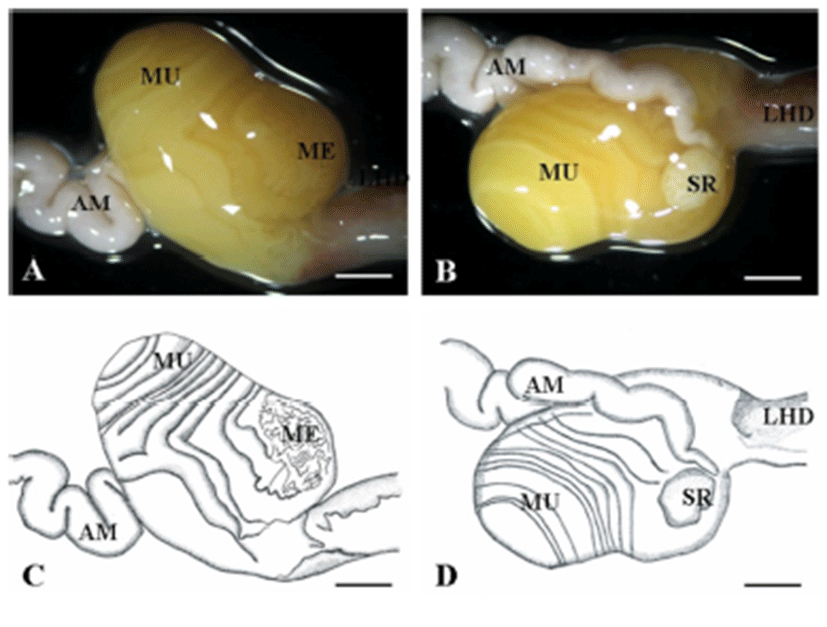
The albumen gland is located in the central part of the accessory genital mass and is yellowish-white color. The albumen gland is tubular (or sac-like) in shape and can be recognized by its granular structure. It is surrounded by a distinct basal lamina (Fig. 6A). The secretory cells have large oval nuclei situated basally, and is filled with spherules 2.4–2.6 μm diameter staining blue-purple in Toluidine Blue, red in PAS, but is not stained Alcian Blue (Fig. 6B to D). Histochemical staining indicates production of neutral mucopolysaccharides in the albumen gland (Table 1).
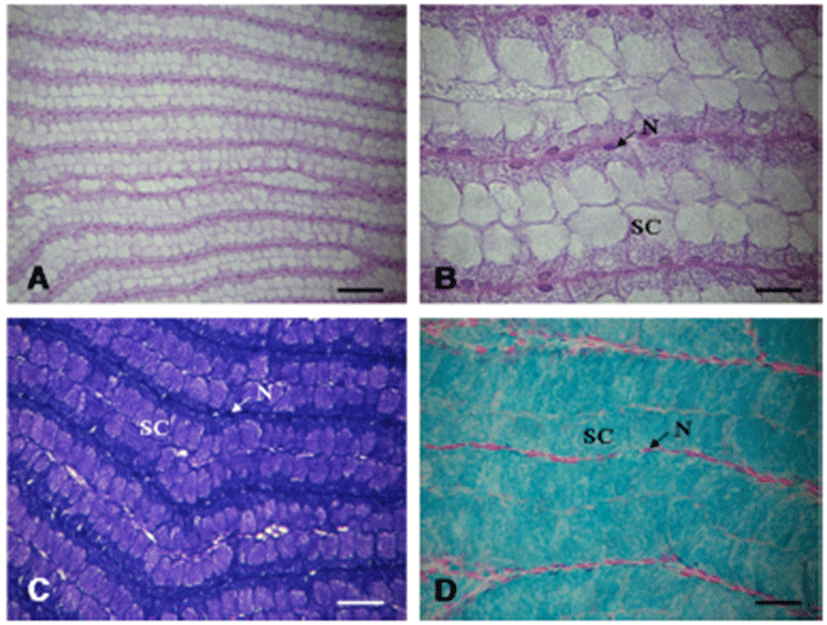
The membrane gland (winding gland) is located to the right of the base of the accessory genital mass and is the shape of a tubule, mostly narrowly coils (Fig. 7A). The secretory cells are distinctly round in shape and contain irregular-shaped granules and basal small nuclei. The wall consists of ciliated cells with extremely long cilia (Fig. 7B). The granules are stained violet with Toluidine Blue (Fig. 7C) and blue in Alcian Blue (Fig. 7D). Histochemical staining indicates production of acidic, sulfated mucopolysaccharides (Table 1).
The mucous gland is white in color and comprised the largest part in the accessory genital mass. The mucous gland is also folded like the albumen gland. Grossly, the mucous gland is observed spiral tubes that encircle the albumen gland (Fig. 8A, B). The granules is stained red-violet in Toluidine blue (Fig. 8C) and blue in Alcian blue (Fig. 8D). Histochemical staining indicate production of neutral and acidic, sulfated mucopolysaccarides (Table 1).
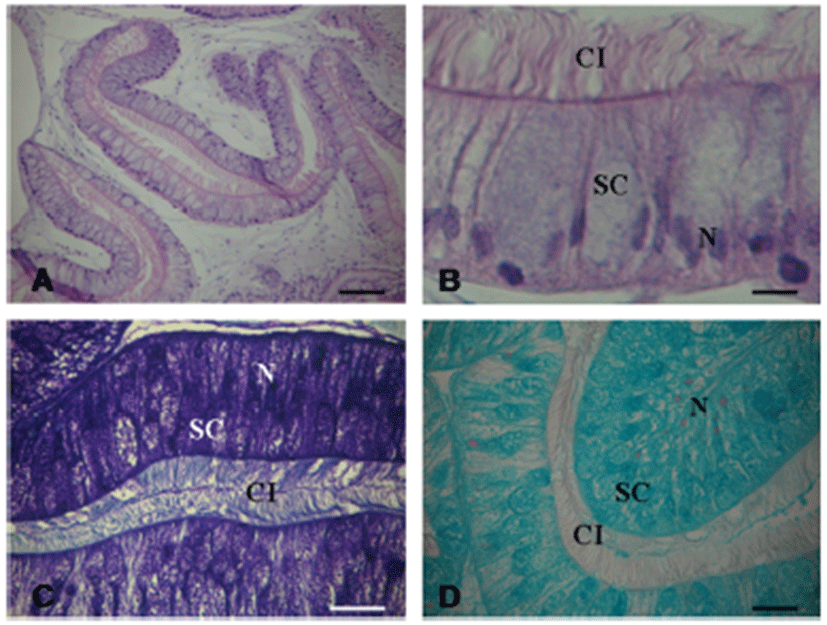
The large hermaphroditic duct is a single tubular gonoduct linking the accessory genital mass to the common genital aperture but consists of two parallel compartments, i.e., the reddish-brown color and thin yellow (or white) color parts, which can be clearly distinguished from each other (Fig. 9A, B). Internally, these two compartments longitudinally divided by internal septum or fold, which are called as the red hemiduct and white hemiduct, respectively (Fig. 9C). The red hemiduct functioned as an oviduct and the white hemiduct functioned as a copulatory duct (Fig. 9C, D).
The RHD is lined by epithelium composed of two kinds of epithelial cells. The first type includes non-ciliated columnar epithelial cells (approximately 70 μm in height and 10 μm in width) which a basal nucleus staining dark with haematoxline. These cells also contain large eosinophilic secretory granules (1–2 μm in diameter). The second type includes irregular-shaped capping cells that cover most of the luminal surface of the RHD. These cells lie between the apices of the columnar epithelial cells and extend long, narrow processes between them; these cells are in contact with the basal surface of the epithelium. These cells have apical cilia (approximately 15 μm long) and nuclei that stain weakly with haematoxyline. The capping cells do not contain large secretory granules, which are detectable by light microscopy (Fig. 10A, B).
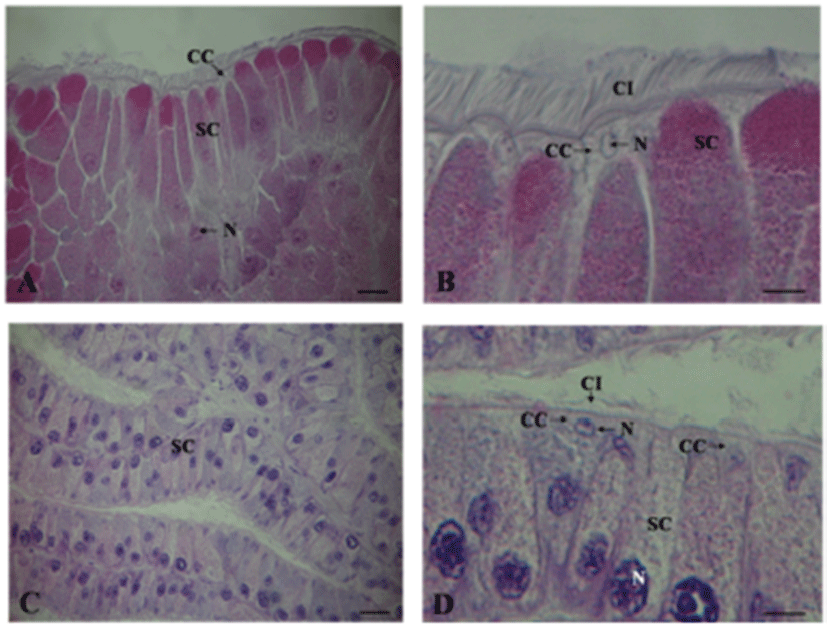
The atrial gland is lined by pseudostratified epithelium composed of non-ciliated columnar epithelial cells and irregular-shaped cpapping cells (Fig. 10C). The capping cells with the apical cilia cover most of the lumenal surface of the artial gland (Fig. 10D).
DISCUSSION
Almost all opisthobranchs are simultaneous hermaphroditic animals that possess structurally and functionally complex reproductive systems consisting mainly of an ovotestis, a complex gonoduct and accessory genital gland. According to the division of gonoduct, the reproductive system is termed as monaulic, diaulic, or triaulic (Ghiselin, 1966). The monaulic type represents a single undivided large hermaphroditic duct and diaulic or triaulic types represent that the large hermaphroditic duct is divided into two or three ducts, respectively. The diaulic type can also be further subdivided into androdiaulic and oodiaulic types (Ghiselin, 1966; Hadfield & Switzer-Dunlap, 1984; Wägele, 2000). This arrangement of reproductive system differs among opisthobranchs and variations can be observed within same family. Nudibranchs and Notaspids generally have diaulic or triaulic systems (Willan, 1987; Cervera et al., 2000; Schrödl, 2000), and Pulmonata and Cephalasidea have monaulic or diaulic systems (Visser, 1988; Kress & Schmekel, 1992). In particularly, aplysiid species have complex and incompletely divided reproductive tracts. Externally, although the tract may appear as a single tube, it is internally divided by at least two incomplete internal folds. Thus, most aplysiids species have either a monaulic or diaulic reproductive system (Ghiselin, 1966; Beeman, 1970). In this study, the reproductive system of A. kurodai was externally a single tube, i.e., monaulic type. However, internal structure of duct is incompletely divided into oviduct and copulatory duct, i.e., the oodiaulic type.
In mature opisthobranchs, the ovotestis is generally the largest of the reproductive organs. It is generally located posteriorly in the body and is closely interdigitated with the digestive gland. The ovotestis is composed of individual follicles or acini that may be widely spaced (Acteonia cocksi; Gascoigne, 1956) or closely packed (Phyllaplysia taylori; Beeman, 1970). In aplysiid, each follicle is covered by a simple epithelial layer and is surrounded by small muscle filaments (Dudek et al., 1980). In many opisthobranchs, oocytes and sperm are produced in the same follicles, however in only a few species, complete separate male and female follicle are found (Reid, 1964), and separate male and female gonads are also found in some Acochlidiacea (Morse, 1976). In this study, A. kurodai also have a single unpaired gonad, the ovotestis, which is composed with numerous follicles. The follicles either partially or completely embedded in the digestive gland tissue, from which they are separated by the basal lamina. Each follicle contains a mixture of both male and female cells in different stage after the onset of sexual maturation. Previtellogenic oocytes lie in the periphery of the follicle, and male cells fill the lumen of follicle; thereafter, the full-grown oocytes are preferentially located in the center of the follicle. This phenomenon is similar to those reported in case of other Aplysia spp. (Beeman, 1970; Dudek et al., 1980). This arrangement may be due to the displacement of preexisting oocytes by the young oocytes.
The small hermaphroditic duct, referred to by some authors as the coelomic gonoduct or ampulla, is a single tube leading from the ovotestis to the fertilization chamber. This duct can be divided into three regions: a preampullar region, an ampulla and a postampullar portion. The autosperm (the animal’s own sperm) formed in the ovotestis is collected and transported to the ampulla, the main part of the small hermaphroditic duct, and mature autosperm are stored prior to copulation (Beeman, 1970). In this study, this duct is narrow in immature individuals, but wider in mature animals and is convoluted to form a seminal vesicle for storage of autosperm. In mature specimens, this tube is creamy-white in appearance due to the presence of sperm. The seminal vesicle is the proximal hermaphroditic duct itself, and not a diverticulum. The autosperm are inactive until ejaculated into a partner. The wall of small hermaphroditic duct is usually composed of ciliated epithelium surrounded by a thin layer of muscle and connective fibers. These results are similar to the results of a previous study regarding characteristics of autosperm and morphology of this duct (Thompson & Bebbington, 1969; Beeman, 1970). The ciliated cells may be responsible for the propulsion of the autosperm and oocytes rapidly through the ampullar during copulation and oviposition.
The accessory genital mass consists of a fertilization chamber and a series of glandular tubes or folds that provide the eggs with the protective layers such as egg capsule and gelatinous matrix. The accessory genital mass has been variously referred to as the female gland mass, anterior genital mass or nidamental glands (Beeman, 1970; Coggeshall, 1972; Klussmann-Kolb, 2001a, b). A three-part accessory genital mass with a proximally located albumen gland, a membrane gland, and a distally located mucous gland is found in most opisthobranchs (Klussmann-Kolb, 2001a, b). However, pleurobranchoidea and nudibranchia possess only two glandular parts (Hadfield & Switzer-Dunlap 1984) and aeolid nudibranchia possess three distinct glandular parts (Gosliner 1994). Aplysia spp. generally presents three glandular parts within the accessory genital mass (Thompson & Bebbington 1969; Beeman, 1970; Coggeshall 1972). In this study, the accessory genital mass of A. kurodai was composed with albumen gland, the membrane gland and the mucous gland. The albumen gland was large, yellowishwhite in color, and its anterior end formed most of the forward part of the accessory genital mass. The membrane gland formed the right-anterior part of the accessory genital mass. The mucus gland was a semi-translucent white organ forming the posterior part the accessory genital mass.
In A. kurodai, the glandular part of the accessory genital mass could be divided into two parts based on their histochemical staining properties and mode of secretion; the basophilic albumen gland (1), so called because the glandular tissue stained blue with toluidine blue and not stained with alcian blue, and the gland contained neutral polysaccharides and the membrane and mucous glands (2), which stained blue with alcian blue and red to violet (acidophilic) with toluidene blue, and the gland contained neutral polysaccharides and acidic, sulphated mucopolysaccharides. These results correspond with those of the histochemical investigation of egg mass in some opisthobranchs (Klussmann-Kolb & Wägele, 2001); it was shown that egg mass of opisthobranchs is composed of various layers, i.e., albumen, albuminous, membrane and mucous layers. This information may provide some understanding of the formation of egg mass in the accessory genital mass.
In simultaneous hermaphrodites, the large hermaphrodite duct is a very complex organ and has variously been referred to as the large, wide, distal or anterior hermaphroditic duct (Hadfield & Switzer-Dunlap, 1984). This duct plays reproductive functions, including transportation of the egg cordon during oviposition, transportation endogenous sperm during copulation as a male, and to receive the penis and exogenous sperm during copulation as a female (Thompson & Bebbington, 1969). Externally, it appears as a single duct in Phyllaplysia taylori, but is actually composed of two distinct and parallel ducts. Internally, these two ducts longitudinally divided by at least two incomplete internal septa or folds, which are called as the spermoviduct and the copulatory or vaginal ducts (Beeman, 1970). Incomplete internal separation of these ducts has been reported in A. fasciata, A. punctata, and A. depilans (Thompson & Bebbington, 1969), and A. californica, A. brasiliana and A. dactylomela (Painter et al., 1985). In the case of A. kurodai species, large hermaphroditic duct was a single tubular which is composed with two parallel compartments, i.e., the reddish-brown and the thin yellow color parts. Internally, this duct was divided with the red and white hemiduct by internal septa. The red hemiduct functioned as a spermoviduct transporting the jelly-coated string of encapsulated eggs. The white hemiduct functioned as a copulatory or vaginal duct carrying endogenous and exogenous sperm.
The atrial gland is a secretory organ located primarily in the wall of the large hermaphroditic duct. Its secretion, when injected into a sexually mature animal, stimulates the process of egg laying (Beeman, 1970; Arch et al., 1980). The atrial gland differs in its shape and location among aplysiids; for example, large hermaphroditic duct of A. brasiliana lacks an atrial gland but has a pea-shaped gland located anterior to the gametolytic gland stalk. A. dactylomela has both a pea-shaped gland with a location and morphology similar to that of A. brasiliana and an atrial gland similar to that of A. californica. In this study, A. kurodai was also observed to possess the atrial gland in the wall of the large hermaphroditic duct. On histological observation, it can be observed that the atrial gland, like the RHD and most secretory areas in the reproductive tract, consists of pseudostratified epithelium which is composed of nonciliated columnar epithelial cells and irregular-shaped capping cells. As in the RHD, the capping cells cover most of the lumenal surface of the atrial gland. The columnar epithelial cells have large eosinophilic secretory granules. This result is similar to the results of the study of other Aplysia spp. (Arch et al., 1980; Beard et al., 1982; Painter et al., 1985). In a previously study on function of atrial gland, Heller et al. (1980) and Strumwasser et al. (1980) reported that the atrial gland might function to temporally link the act of egg laying with copulation and thus, secrete peptides into the hemocoel in response to mechanical or chemical stimulation by the penis. However, morphological and physiological evidence indicates that the atrial gland is an exocrine organ that secretes into the lumen of the large hermaphroditic duct rather than into the hemoceol (Arch et al., 1980; Beard et al., 1982). In the present study, the atrial gland was confirmed to be an exocrine organ located in the wall of the large hermaphroditic duct and was presumed to play a direct role in oviductal function of large hermaphroditic duct.

