INTRODUCTION
Dialkyl phthalates are widely used in plastics, hugely synthesized chemicals, and exposed ubiquitously to human. Until early 2000s more than 18 billion pounds of phthalates are used every years (Bizzari et al., 2000; Blount et al., 2000; Guo et al., 2011). Di(2-ethylhexyl) phthalate (DEHP, CAS NO: 117-81-7) is one of the most popular phthalates: it has been used from 1930s and the production volume is about 2 million tons in 1999 (Center for the Evaluation of Risks to Human Reproduction, 2000). In general population, the urinary phthalate metabolites are detectable (Silva et al., 2004). Because DEHP is a suspected endocrine disrupting chemicals (EDC) in human, its amount are regulated for manufacture the industrial products in almost countries (for example, 800 mg/kg in REACH SVHC, 0.1 wt% in USA, 1,000 mg/kg in Hongkong). However, it is still used to make for many commercial products including sprays, toy, raincoats, lubricants, cosmetics, and medical devices such as blood bags and dialysis equipment (Liu et al., 2017).
Phthalates show toxicity in human and wild animals (Kavlok et al., 2002). It is known that the toxicity of phthalate is dependent on the chemical structures and administered dosage, and administered life stage (Parks et al., 2000; Foster 2006). DEHP has the most potent toxicity in reproduction among phthaltes (Heindel et al., 1989; Davis et al., 1994). In human, female factory workers exposed chronically to high levels of phthalates is associated with decreased rates of pregnancy (Aldyreva et al., 1975). Interestingly, in vivo, 2 g/kg DEHP causes anovulation in adult cycling rats (Lovekamp-Swan & Davis, 2003). Numerous studies were performed with phthalate and revealed the anti-androgenic effects in male rat at medium and from 10 mg/kg/day levels in depend on the age (Jarfelt et al., 2005; Christiansen et al., 2010). However so for confused and controversy about the effects of such exposure. An epidemiological study in US EPA could not find the relationship between reproductive toxicity and phthalate (ACEIII, 2013). Someone suggested that in these epidemiological studies the range of dose is much lower than previous toxicological studies (Lovekamp-Swan and Davis, 2003; Jarfelt et al., 2005; Andrade et al., 2006), but others ask new viewpoints (Rhomberg & Goodman, 2012; Vandenberg et al., 2012; Zoeller et al., 2012).
Previously, the Agency for Toxic Substances and Disease Registry (ATSDR) estimates that the maximum daily exposure to DEHP for the general population is about 2 mg/day. So the low dose of phthalate is permitted. Controversy for the review of ACEIII (2013), a few studies showed toxicity of low dose phthalate in male infertility and steroidogenesis (Bloom et al., 2015; Savchuk et al., 2015). Recently it become a big issue to study the effect of very low dosage EDC with long period (Kawaguchi et al., 2015; Xu et al., 2017). In addition, so far, it is largely masked the effects of DEHP during reproductive period. Therefore in this study, the effects of very low dose DEHP on the body weight, reproductive organ weight and reproduction were evaluated in reproductive period female mice and male employed the OECD test guideline 443 “the expended one-generation reproductive toxicity study” for parents.
MATERIALS AND METHODS
CD-1 mice (10–12 weeks old) were used for parental group. All experimental animals were studied according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health under the Experimental Animals Committee of Sungshin Women’s University. They were maintained under controlled temperature (22–24°C), humidity (45–55%) and light (14 h light/10 h dark) conditions and were fed phytoestrogen free diet (2018 Teklad global 18% protein rodent diet; ENVIGO, Madison, WI, USA). and water (free or DEHP containing) ad libitum using glass bottles. Pregnant mice were allowed to deliver their pups naturally and were weaned at 21 post-natal days.
Di(2-ethylhexyl)phthalate (DEHP Sigma Aldrich Cat# 36735) was selected as a endocrine disrupting chemicals and used at two concentration based on Niermann et al. (2015): 133 g/L and 1,330 /g/L in drinking water. 10 female and 10 male mice were involved in each groups. The chemical stock solution was prepared in purified water and stored at -20℃. Administration was proceeded to parents according on schedule suggested by OECD test guideline 443. Briefly, animals were administered in drinking water containing DEHP during 10 weeks (pre-mating; 2 weeks, mating; 2 weeks, post mating included pregnancy and lactation; 6 weeks) and then were anatomized for organ sampling.
The control and experimental groups of the same sex were sacrificed at the same time. The males and females mice were sacrificed at end of chemical treatment periods for 10 weeks, and the reproductive organ (testes and epididymis in males; ovary and uterus in females) were excised out and weighed individually. Relative organs weight was calculated based on organ to body weight.
The number of sperm was counted with Makler counter chamber (Sefi Medical Instruments LTD, Santa Ana, CA, USA). The caudal sperm collection was followed the method for mouse in vitro fertilization (Hogan et al., 1994).
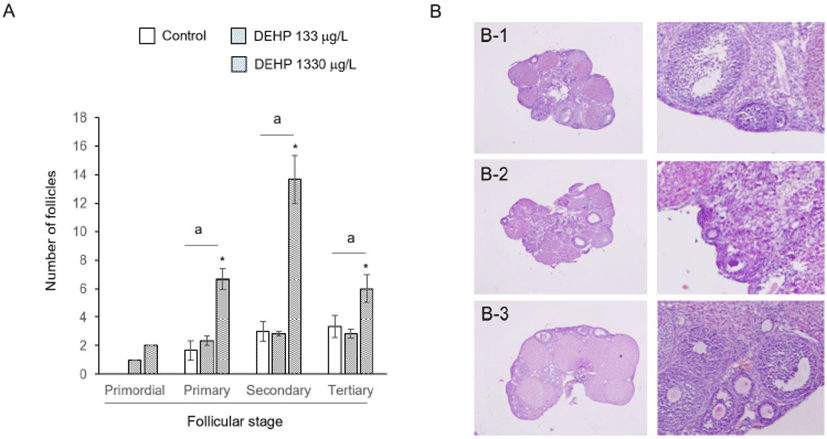
Extracted ovaries were weighted with electronic balance and fixed with 4% paraformaldehyde in PBS. And then the paraffin embedded ovaries were sectioned for histological studies (4 Em thickness). Sections were stained with Hematoxylin and Eosin. The 13th section of serial section from the surface were used for follicle count. Follicle stage was defined as primordial, primary, secondary, and tertiary follicle (Rybska et al., 2018).
RESULTS
Within the examined dosage range, the weight of testis or epididymis were not different from them of the control (Table 1). In female mice, the ovarian weight significantly decreased in DEHP 1,330 Wg/L drinking group (Table 1). The uterine weight also decreased in DEHP 1,330 eg/L drinking group (Table 1).
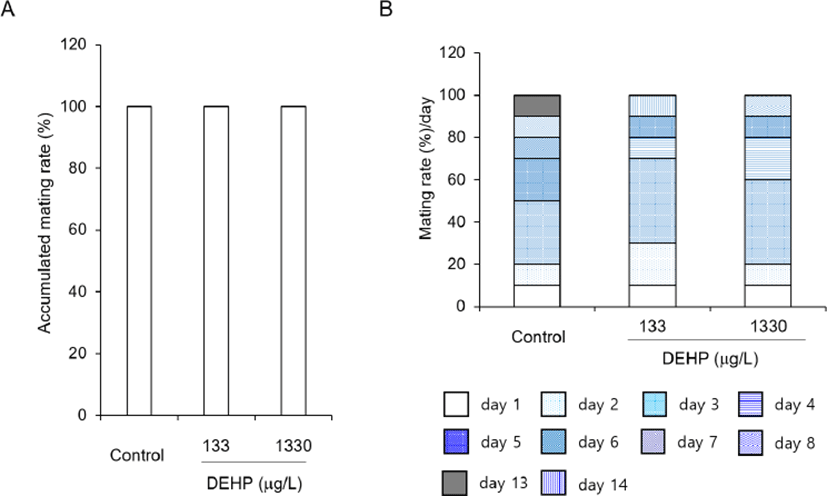
The mice which were pre-administered with DEHP for 2 weeks before attended reproduction, were housed for 2 weeks to know the effects of DEHP on the reproduction. The accumulated mating rates were not different between groups (Fig. 1A) and the mating rates at estrus stage were not changed by DEHP administration (Fig. 1B).
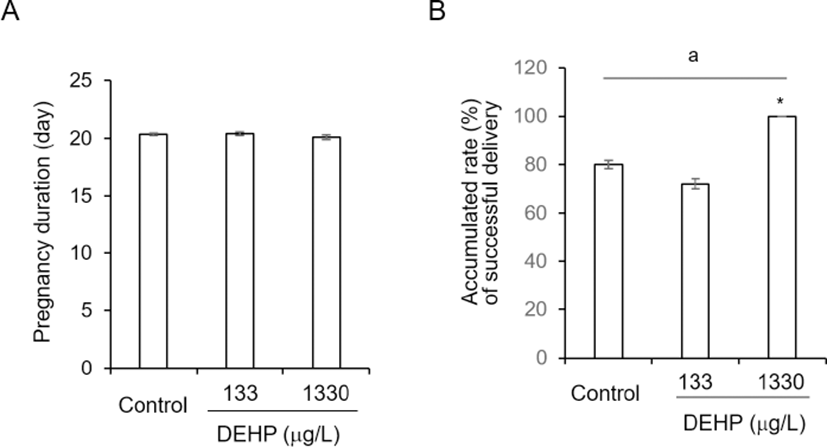
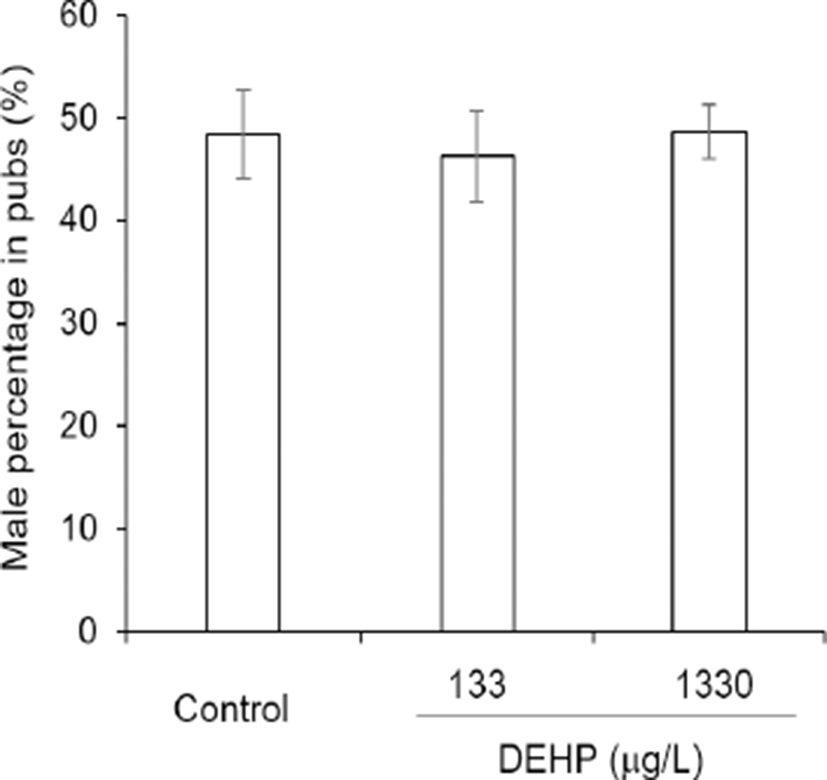
The pregnancy duration was from 20.10±0.216 to 20.35±0.109 and not statistically different between groups (Fig. 2A). On the other hand, the successful delivery rate was significantly high at DEHP 1,330 tg/L drinking group compared with others (Fig. 2B). However, the sex ratios of pubs were not different between groups. The percentages of male among pubs were 48.46±4.328 at control, 46.29± 4.397 at DEHP 133 4g/L, and 48.74±2.624 2g/L (Fig. 3).
The litter sizes were different between groups. As seen at Table 2, it was dramatically increased in DEHP 1,330 ig/L drinking group (p<1E-8).
| Generation | Group | No. female | Litter size |
|---|---|---|---|
| P0 | Control | 10 | 9.090.71 |
| DEHP 133 | 10 | 8.081.48 | |
| DEHP 1330 | 10 | 11.610.88 |
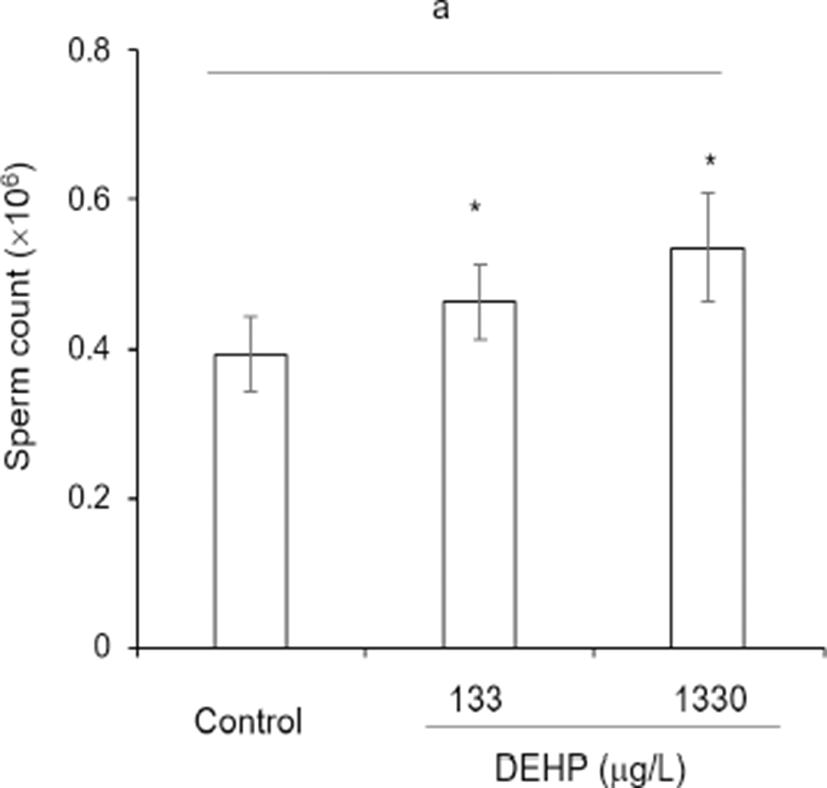
To evaluate the possible role of DEHP on the litter size, the number of caudal epididymal sperm and the follicles in ovary was counted. The numbers of sperm were increased concentration-dependently by administration of DEHP. The ratio of sperm count to body weight in caudal epididymis was 0.39±0.051, 0.46±0.050, and 0.54±0.073, respectively at control, DEHP 133 0g/L, and DEHP 1,330 g/L (Fig. 4).
The large number of follicles were detected at DEHP 1,330 g/L drunk mother group. The numbers of primary, secondary, and tertiary follicles were significantly many more compared to control and DEHP 133 g/L drunk mother groups (Fig. 5A). It is hard to find the primordial follicles in germinal epithelia of control sections (Fig. 5B).
DISCUSSION
Previously we reported that very low-dose chronic administration of nonylphenol can work as endocrine disruption (Cha et al., 2017) and other groups also suggest the possible role of low-dose EDC chemicals in physiological levels. For example, exposing very low DEHP from gestation day 11 to birth increases preantral follicle numbers and caused of some breeding abnormalities (Niermann et al., 2015). Four weeks drinking of 50 and 500 tg/L NP has multigenerational effect on selective reproductive organ (Kyselova et al., 2003). Therefore, now the studies at the level of physiology become more important (Vandenberg et al., 2012; Zoeller et al., 2012).
Many studies have been in relatively high dosage (Lovekamp-Swan & Davis, 2003; Christiansen et al., 2010). Reproductive and developmental toxicity studies did in the female rodent during organogenesis (Kaul et al., 1982). In mouse toxicity is dose-dependent and timing of exposure (Tomita et al., 1986). DEHP administration in adult rats induce hypoestrogenic anovulatory cycles and polycystic ovaries (Davis et al., 1994; Lovekamp-Swan & Davis, 2003). DEHP induce atresia of tertiary follicles (Grande et al., 2007) and cause of endometriosis and uterine leiomyoma (Weuve et al., 2010). However, interestingly, the low-dose chronic administration of DEHP had opposite results from the previous reports conducted with high dosage.
In this study, the litter size was significantly big and the accumulated rate of successful delivery was increased dramatically at 1,330 eg/L DEHP drunk mother compared with control. On the other hand the weights of ovary and uterus were decreased at this condition. These results is inconsistent to the known general role of EDC. Such a phenomena also reported other groups with another ECDs (Vandenberg et al., 2012).
The weight of ovary and uterus were decreased by administration of DEHP. In this study, the reasons of such results is not clear and needed further studies. In a dose-dependent manner MEHP decreases granulosa cell aromatase expression (Lovekamp-Swan & Davis, 2003). In human based studies, the phthaltate metabolites are revealed the main cause of reproductive abnormality as phthalate. Infertility risks are increased in MEHP dose-dependent manner in Chinese men (Liu et al., 2017). It has been suggested that such results from the antagonist effects of MEHP in vitro (Stroheker et al., 2005). However, it is needed further study how its effects are arise from what DEHP metabolite after absorption into the body. Because these metabolite has been suggested as a real reason of the DEHP toxicty (Gray & Beamand, 1984).
Interestingly primary, secondary, and tertiary follicles were significantly many more at DEHP 1,330 eg/L drinking groups than the control. On the other hand, the sperm count also increased in DEHP drinking male groups compare to control. These interesting phenomena are first time discovery. The study of Niermann et al (2015) also showed the increase the preantral follicle number of low DEHP (exposed from gestation day 11 to birth) but the exposing period is different from this study. The suggested mechanisms which affects reproductive function are various including follows: an anti-androgenicity (Latini et al., 2003; Akingbemi et al., 2004), suppressor of the expression of steroidogenesis related factors and activation of PPARs (Borch et al., 2006; Howdeshell et al., 2007; Wilson et al., 2008), activator of caspanse-3 in specific cells through deacetylation of SP3 (Guida et al., 2014), and inducing agent of inflammation (Ferguson et al., 2012). Recently a suggested mechanisms of phthalate is the role of low dose of phthalate as androgen effects on steroidogenesis but high doses as antiandrogen action (Fan et al., 2010; Li et al., 2016; Liu et al., 2017).
From this study we firstly report the effect of low-dose chronic administration of DEHP with drinking could change the ovarian follicle population. Though further studies are needed to identify what are the mechanism of DEHP in folliculogenesis. In addition, it is also needed the studies why the number of sperm is increased DEHP. Put together, those finding is different from previous high-dose effects and suggest the physiological role of DEHP in gonads.

