INTRODUCTION
The marine medaka, Oryzias dancena (Beloniformes; Teleostei) is a euryhaline teleost that mainly inhabits the brackish or freshwater of river mouths and estuaries around Bengal Bay and the Malay Peninsula (Roberts, 1998). In hyperosmotic environments, the marine medaka showed better tolerance than the Japanese medaka, O. latipes, including survival rates of adult fish and hatching rates of oosperm (Inoue & Takei, 2003; Kang et al., 2008). Detailed information on marine medaka biology has begun to be exploited, especially regarding early gonadogenesis, sex differentiation, early ontogenesis, and embryogenesis (Song et al., 2009a, 2009b). In addition, Cho et al. (2010) researched tolerance capacity to salinity changes in this species. This species is highly capable of hyper-osmoregulation as well as hypo-osmoregulation. Recently, the anesthetic effects of clove oil and lidocaine-HCl on marine medaka were reported by Park et al. (2011). Anesthetic effects on experimental groups of different sizes of marine medaka were observed, and both anesthetic time and recovery time for the juvenile group were shorter than those of the adult group. Kim et al. (2011) reported the characteristics of two functional glucocorticoid receptors in this species.
Triploidization is a technique used to generate sterile aquatic animals by taking advantage of the incompatibility in pairing the three homologous chromosomes during meiosis I (Don & Avtalion, 1986; Park et al., 2016b). This technique has also been used to enhance the productivity of several fish species because of its assumed ability to increase yield by channeling the energy required for gonadal development into somatic growth (Tave, 1993; Park et al., 2016a). More importantly, it generates fish that are unable to breed but contribute to the local gene pool if they were to accidentally escape from confinement. By conferring sterility of exotic fish for a limited purpose, triploidy can serve as an effective method for reducing or eliminating the environmental risks of genetically modified organisms (Dunham & Devlin, 1999).
Fluctuating asymmetry occurs when the traits of one side of a supposedly bilaterally symmetrical organism differ in a random way from those of the other sides (Wilkins et al., 1995). It is shown as a difference in the number, size, shape or other features of the trait between the left and right side. Developmental homeostasis normally maintains fluctuating asymmetry at a low level. When fluctuating asymmetry increases, it is thought to show a destabilization of development, either by endogenous genetic factors or by exogenous factors such as environmental stress. There are considerably numerous debates over the precise relationship of genetic factors and developmental stability as shown in fluctuating asymmetry, with two major hypotheses (the heterozygosity hypothesis and the genomic coadaptation hypothesis) being distinguished (Wilkins et al., 1995). The former represents that highly heterozygous individuals will have greater developmental stability and therefore lower fluctuating asymmetry, than homozygous individuals because heterozygosity may buffer the organism from perturbations affecting the normal developmental processes and pathways. The latter represents that it is coadapted gene complexes (Wilkins et al., 1995) that ensure developmental stability and when such complexes are disrupted, for example by hybridization or gene flow, stability decreases and fluctuating asymmetry is elevated. In this study, we have examined fluctuating asymmetry of eye diameter, maxilla length, operculum length, and the number of pectoral fin ray and pelvic fin ray between ploidy and sex in marine medaka.
MATERIALS AND METHODS
The experimental group of diploid marine medaka, Oryzias dancena in this study was reared by methods of Park et al. (2011). On 24 September 2017, one hundred fish were quarantined by the male and female categories and habituated in the 100 L glass aquariums for 3 days. The sex ratio of males and females was 60 males and 40 females. The culture water was dechlorinated and 30% of water in the aquarium was exchanged every day. Artemia collected from the cultured aquarium were provided to fish every day. To collect eggs, the fish whose standard length was over 25 mm, was used in this experiment and 35 males and 15 females of marine medaka were placed in each of two aquariums, and 1,000 fertilized eggs were collected immediately with net. The fertilized eggs of diploid experimental group (n=500) were reared in 100 L glass aquarium.
The fertilized eggs of triploid experimental group (n=500) were left to fertilize for 5 min, and were subjected to cold-shock treatment (4℃) for 60 min in order to prevent the onset of the second polar body (Park et al., 2016b, 2018). Afterward, the treatment eggs were reared in 100 L glass aquarium. After 2 months, flowcytometric analysis was performed to estimate the average cellular DNA content of all individuals from triploid marine medaka. After anesthetizing the fish with 100 ppm clove oil (Park et al., 2011), sample tissues for analysis were separated from all individuals of tail fin. One million of tail fin cells were collected and stained using a high-resolution DNA staining kit (Partec GmbH, Germany) under dark condition at room temperature for 15 min (Park et al., 2018). Stained samples were analyzed on Partec PA-II flowcytometer (Partec GmbH, Germany) to determine the relative DNA content. The red blood cells (2.8 pg DNA/nucleus) of mud loach, Misgurnus mizolepis were used as a standard reference. Individuals which were found triploid were quarantined in a 30 L glass aquarium for experiment (Park et al., 2018).
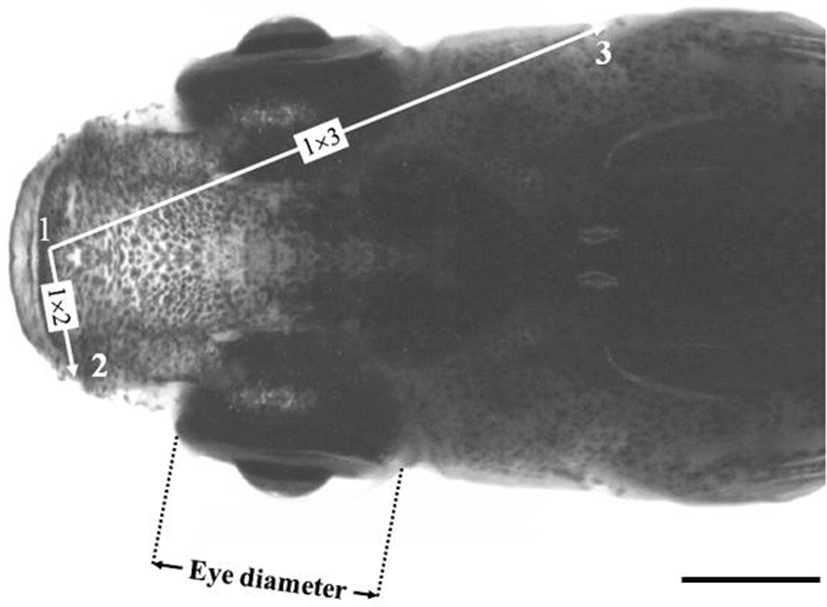
On 20 May 2017 (270 days after hatched), this study was performed using samples of 100 marine medakas in total, 50 males and 50 females in diploid and triploid, respectively. Mean standard length of used samples was 3.21±0.49 cm, and mean body weight of used samples was 584.1±11.67 mg. Using the method of Park et al. (2011), the samples were anesthetized with 100 ppm clove oil at 26℃. For the measurement of fluctuating asymmetry, anesthetized samples were fixed in 10% neutral formalin. To avoid the sampling fish with guts that were distended by large quantities of food, fish were left to starve for 24 hrs before sampling (Park et al., 2001). Fluctuating asymmetry measurements of fixed samples were taken to the nearest 0.1 cm using digital vernier calipers (CD-20 CP; Mitytoyo, Kawasaki, Japan). As shown in Fig. 1, eye diameter indicates horizontal distance, and maxilla length (length from middle point of maxilla to each tip of maxilla: 1×2 in Fig. 1), and operculum length (length from middle point of maxilla to each operculum: 1×3 in Fig. 1) indicates direct distance.
Digital pictures were taken for fixed samples of each group using a microscope camera (Axiocam MR, Carl zeiss, Germany; n=100). The Axioskop 4.1 image analysis software (Carl Zeiss, Germany) was used to measure meristic characteristics. Meristic characteristics of pectoral fin ray and pelvic fin ray were measured using the pictures. The differences between the groups were analyzed by student’s t-test using the SPSS statistics package (SPSS 9.0, SPSS Inc., Chicago, IL, USA). Every experiment was conducted in triplicate.
RESULTS
Table 1 shows the mean value of meristic and morphometric asymmetry in male marine medaka, Oryzias dancena, and results of t-test for difference between diploid and triploid. Eye diameter and maxilla length showed no significant difference between left side and right side in diploid and triploid groups. In diploid and triploid group, operculum length of left side was significantly longer than that of right side (p<0.01). In diploid group, the number of pectoral fin ray’s left side outnumbered the pectoral fin ray’s right side by ten to nine (p<0.05). Trend of pectoral fin ray’s number in triploid group showed opposite disposition with that of pectoral fin ray’s number in diploid group (p<0.01). Number of pelvic fin ray in diploid group showed no significant difference between left side and right side. However, the number of pelvic fin ray in triploid group has significant disparity between left side and right side, and the number of pelvic fin ray’s right side outnumbered the pelvic fin ray’s left side by six to five (p<0.05).
Table 2 shows the mean value of meristic and morpho-metric asymmetry in female marine medaka, and results of t-test for difference between diploid and triploid. Eye diameter and maxilla length showed no significant difference between left side and right side in diploid and triploid groups. Operculum length of diploid group showed no significant difference between left side and right side. In triploid group, operculum length of left side was longer than that of right side (p<0.05). In diploid group, the number of pectoral fin ray’s left side outnumbered the pectoral fin ray’s right side by ten to nine (p<0.05). Results of pectoral fin ray’s number in triploid group were similar to those of pectoral fin ray’s number in diploid group (p< 0.01). Also, the number of pectoral fin ray in triploid group had one more than that in diploid group. The number of pelvic fin ray in diploid group showed no significant difference between left side and right side. However, the number of pelvic fin ray in triploid group has significant disparity between left side and right side, and the number of pelvic fin ray’s left side outnumbered the pelvic fin ray’s right side by six to five (p<0.05).
In diploid and triploid group, eye diameter and maxilla length’s trends of female marine medaka were similar to those of male marine medaka (Tables 1 and 2). Operculum length of diploid male group showed significant difference between left side and right side, but that of diploid female group showed no significant difference between left side and right side. Results of operculum length in triploid male group displayed similar propensity with those of operculum length in triploid female group. Findings of pectoral fin ray’s number in diploid male group showed similar propensity with the results of pectoral fin ray’s number in diploid female group, however, the pectoral fin ray’s number in triploid male group showed consinderable difference with that of pectoral fin ray in triploid female group. Also, the results of pelvic fin ray’s number in all groups showed similarity with those of pectoral fin ray’s number in all groups.
DISCUSSION
According to Soule (1967), if heterozygosity or genomic coadaptation mediates developmental stability, then populations which are highly asymmetrical in one character are likely to be asymmetrical in other characters. On this basis, asymmetries in different traits are often combined to make an overall population asymmetry parameter (PAP; Soule, 1967). The meristic and morphometric traits analyzed here appear to represent two distinct characteristic sets. The meristic traits showed elevated fluctuating asymmetry in hybrids but these decreased when hybrids became triploid. The morphometric traits did not show elevated fluctuating asymmetry in hybrids and were relatively unaffected by triploidisation. There was no consistent, significant correlation to fluctuating asymmetry in those two characteristic sets among individual salmon or individual hybrids.
In the previous paper, Claytor and Verspoor (1991) has found that variation in meristic and morphometric characteristics of sympatric Atlantic salmon, Salmo salar populations was discordant and variations in them were likely to be congruent only by coincidence. It seems reasonable to conclude that the expression of these characteristic sets in salmon is under separate genetic control. This is consistentistic with the observation that meristic traits are determined during embryonic development before hatching (Taning, 1950; Barlow, 1961) whereas morphometric traits remain unstable during later growth, also their phenotype is strongly influenced by rearing conditions, especially stream gradient (Claytor et al., 1991). Therefore, it seems unacceptable, at least as far as Atlantic salmon is concerned, to combine those two kinds of traits in analysis of fluctuating asymmetry as is done with other species (see, for example, Soule 1967; Graham & Felley, 1985).
In our study, triploid marine medaka, Oryzias dancena was significantly different from that of diploid in some factors of fluctuating asymmetry. According to Wilkins et al. (1995), triploid salmon was not significantly different from their diploid siblings in fluctuating asymmetry, although their overall values were lower. Consequently, the presence of the extra chromosomal set had no significant effect on developmental stability. In triploid hybrids, in contrast, duplication of the maternal set of salmon chromosomes restores the relational balance absent in diploid hybrids, resulting in a statistically significant reduction in fluctuating asymmetry and accordingly greater developmental stability. In their study of rainbow trout, Oncorhynchus mykiss, Leary et al. (1985) also observed a significant reduction in fluctuating asymmetry due to triploidisation (Wilkins et al., 1995). Future investigations of asymmetry in marine medaka are sure to focus on the directional asymmetry of gonad development between male and female diploid marine medaka.

