INTRODUCTION
Hippocampus fish (Gasterosteiformes; Syngathidae) live mainly in shallow coastal areas with many coral reefs in temperate and tropical waters. They have a unique spawning method in which females hand over the eggs to males’ marsupium in the spawning season and males give birth (Foster & Vincent, 2004; Lourie et al., 2004). Hippocampus kuda was introduced as a new species by Bleeker in 1852, but recently Lourie et al. (1999a), it was confirmed that it was the same species as the true Hippocampus, H. aterremus, published by Jordan and Snyder (1901).
It is known to be distributed in India and the Pacific, Pakistan, Hawaii, Society island and the coast of Japan (Fishbase, 2018), It is reported that there are 52 genera and 215 species in the world and 6 genera and 11 species in Korea, which belong to Syngnathidae. These, 25 species are known in Hippocampus, which is seahorse (Chyung, 1977; Myers, 1979; Vari, 1982; Kim et al., 2005), but in recent years, seahorse has been re-identified as having 32 species in seven habitat marine categories using external morphological traits and genetic traits (12, 16S mtDNA) based on coastal category ecological features with limited swimming waters (Lourie et al., 1999b; Koh et al., 2004).
The demand for sea horses rapidly increased due to the use of ornamental organisms and herbal medicine materials, resulting in a tremendous decrease in resources in natural waters such as Southeast Asia due to over fishing and therefore, some species are endangered (Vincent, 1996; Lourie et al., 1999b). Since the survival rate of early fish larvae is very low as well as brood stock culture, artificial seed production is difficult, and studies on species conservation are very urgent (Scarrat, 1995; Vincent, 1996).
The coastal area of Korea where seahorses mainly live is shallow and consists of eelgrass bed. However, since the habitat is gradually decreasing due to marine pollution and coastal landfill, it is necessary to protect the habitat of sea horses which are sensitive to environmental change and have low mobility.
Studies on seahorses include studies on increased resource conservation and early life history such as mating habit (Masonjones & Lewis, 1996), spawn behavior (Woods, 2000a; Masonjones, 2001), breeding environment and food creatures (Hilomen-Garcia et al., 1999; Payne & Rippingale, 2000; Woods 2000b; Adams et al., 2001), H. kuda (Koh et al., 2004), H. barbouri (Choi et al., 2006a), H. coronatus (Choi et al., 2006b), and H. histrix (Van Look et al., 2007) etc., Koh et al. (2004) reported that the development of the philippine H. kuda has not been studied.
Every species of seahorses are similar in the number of dorsal fin rays, development of the body's bony plate ring, body protrusions and tail skeleton during the period of fish larvae. In the case of adult fish, the body color changes according to the environment and growth, and body protrusions fall off easily, so it is known that species are difficult to be classified morphologically with possibility of interspecific hybridization (Koh et al., 2004). Therefore, in this study, we observed the courtship, fertility behavior and larvae and juveniles of external developmental of the reproductive H. kuda, which were known to be H. aterremus in the past. Seed production and species preservation studies will be used as baseline data for taxonomic studies.
MATERIALS AND METHODS
The samples used in this study were 20 pairs of male and female H. kuda taken at Wonju Island in Goheung-gun, Jeollanam-do in June 2015. After transported to the laboratory, they were placed in a glass square bath (30× 90×60 cm) to observe mating behavior. Temperature and salinity were maintained at 27.5°–28.5° (mean 28.0°) and 34.5–35.5 psu (mean 35.0 psu), respectively. Small ice shrimps (Hikari, China) were fed twice a day.
For observation of bearing, hatched males were breeding management. For observation of egg shape, hatched eggs were taken, observed and measured using a universal projector (Nikon JP V-12B, Japan) and a stereomicroscope (Nikon SMZ18, Japan). For observation of larva and juveniles morphological development, ten individuals were collected immediately after bearing and fixed in 10% of neutral formalin, and a universal projector and a stereomicroscope were used to measure the external characters up to 0.01 mm.
The breeding environment was the same as that of their mothers, and Brachionus plicatilis was feed from 12 to 21 days after bearing and Artemia sp. nauplius incubation larva was supplied from 21 to 41 days after bearing. After that, small ice shrimps were supplied in small quantities.
RESULTS
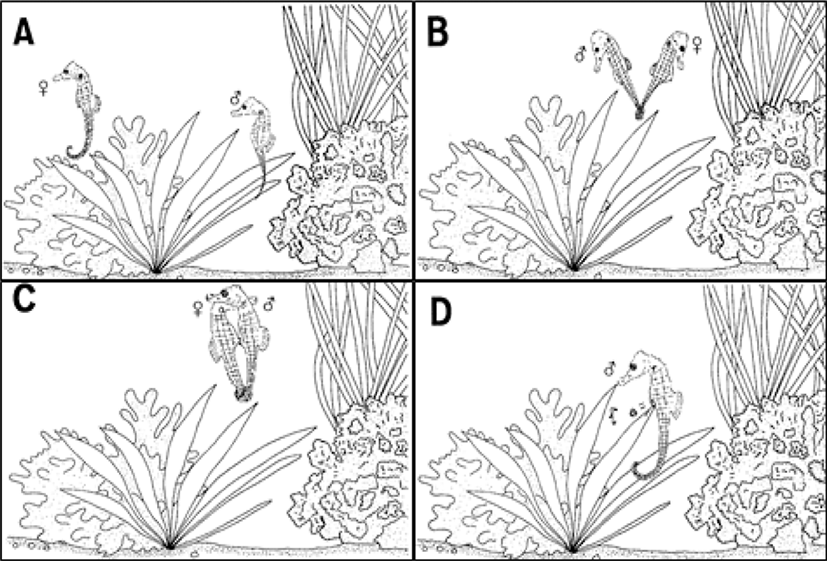
The mating behavior of H. kuda is contact behavior that males and females face each other and hit the snout, and males showed continuously following actions (Fig. 1A). The female who is about to spawn projected the oviduct in the abdomen and then, the female reacted to the mating behavior of the male, and the male wound its tail of the female and swam (Fig. 1B). The male and female swiftly swam to the top of the water with their tails winding, and they repeatedly performed the same action, and the female layed eggs by inserting its oviduct into the male's marsupium (Fig. 1C). The male that spawn protected the fertilized eggs in its marsupium and they began to hatch slightly from the 12 days after fertilization. The male marsupium was swollen over time. On the 19 and 20 days after the fertilization, the male gave birth to about 70 larvae from its marsupium with its tail wrapped around the seaweed (Fig. 1D).
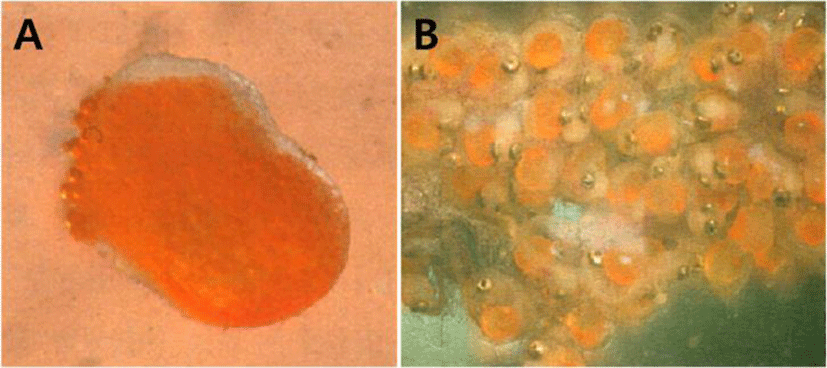
The amount of spawn in H. kuda females was 112–162 (mean 143, n=10), and the amount of spawn identified in the male's marsupium was 82–136 (meane 112, n=10), and the eggs of the male's marsupium accommodated about 80% of the female spawn amount.
The egg shape was similar to the peanut shape, the color was orange, and the eggs had some stickiness. The male's marsupium had wrinkles on the inside of the pouch, sponge- shaped epidermis and small veins were observed, and there were several eggs on the honeycomb-shaped skin tissue (Fig. 2A, B).
Immediately after bearing of H. kuda, larva and juveniles were 6.97–8.81 standard length (SL) mm (mean 7.89± 1.30 mm, n=10), and mouth and anuse were open, and the fin of each part was developed. The number of fin rays is 15–18 dorsal fins and 8 pectoral fins. At head, one strong thorn was developed over the eyeball, and two thorns were developed on the gill lid, and it was observed that the thorns were split into two according to the individuals. At this time, the larvae and juveniles completely absorbed the egg yolk and the body surface part in front of the anus was transparent compared to the other body surfaces, and no chromophore was observed. The number of body's bony plate ring was 10–11+21, and body's bony plate ring was developed around the myotomes part (Fig. 3A).
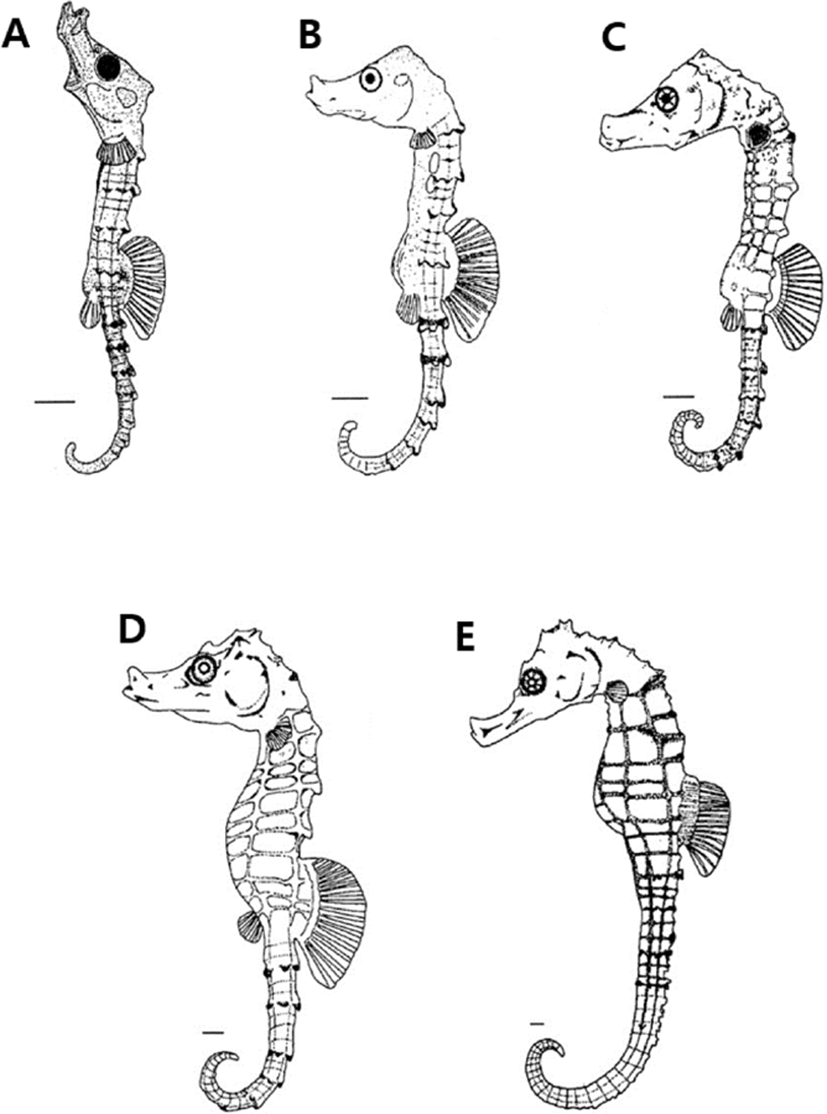
On the 4 days after bearing, larvae were 7.02–9.47 SL mm (mean 8.24±1.73 mm, n=10), the number of pectoral fins increased to 11, their snout was elongated, and the black vesicles formed on the body surface became thicker like leaves. The nostrils began to open in front of the snout, a pair of safety thorns appeared around the eyes, and the distal end of the thorns developed inward (Fig. 3B).
On the 12 days after bearing, larvae were 8.91–11.2 SL mm (mean 10.0±1.61 mm, n=10), and the number of body's bony plate ring on the trunk was 11, on the tail of them was 33–36. A pair of thorns were formed at the edge of the back of the head, and the shape of nostrils began to develop in a shape similar to dumbbells. The shape of the snout was similar to that of adult fish, showing a feeding pattern of sea horses taking in prey by sucking seawater with the mouth (Fig. 3C).
On the 21 days after bearing, larvae were 12.1–14.8 SL mm (mean 13.4±1.90 mm, n=10), and the thorns on the upper part of the head and back were elongated, and the black vesicles deposited on the head and gill lid were widely deposited (Fig. 3D).
On the 41 days after bearing, juveniles were 17.1–17.8 SL mm (mean 17.4±0.49 mm, n=10), and the black vesicles deposited on the fins of each part were more intensely deposited in the lower part and were evenly distributed overall. The five black radial strips formed in the eyes were irregularly colored depending on the individual, and the thorns formed around the eyes were 2–3, with the ends sharply divided, and overall external morphology and number of body's bony plate rings are 11+33–36. And the number of fin rays is 15–18 dorsal fins and 13–14 pectoral fins, which are the same as those of adult fish (Fig. 3E).
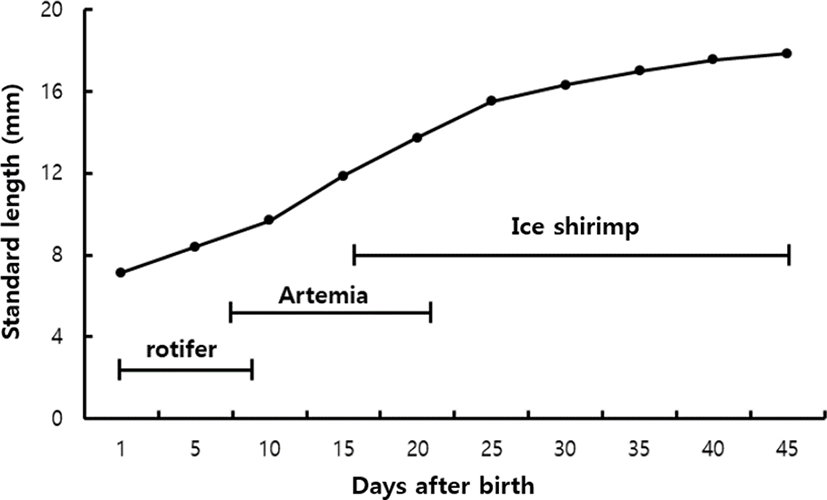
Growth changes were observed from immediately after bearing to 45 days. As a result, larvae started to ingest rotifer immediately after bearing and showed a moderate growth until 10 days after bearing. As the mouth developed, the intake of the Artemia larvae became active and showed rapid growth until 25 days after bearing. After the reduction of the supply of Artemia and the sequential supply of small ice shrimps, the growth was moderate until 45 days after bearing (Fig. 4).
DISCUSSION
Seahorse is a coastal sedentary fish that is known to have a spouse in the same habitat, but molecular biological considerations for phylogenetic classification suggest that many interspecies crosses occur, necessitating reconsideration of taxonomic criteria (Vincent & Sadler, 1995; Lourie et al., 1999b). It is a species that is difficult to classify among species because the development of larva and juveniles period is similar. On the other hand, it has a phylogenetically close relationship with the Syngnathidae fish, which is a closely related species. However, their larva and juveniles are easy to be distinguished from those of other fishes because they display external characteristics close to the adult fishes immediately after bearing (Gill, 1905; Mi et al., 1998).
Generally, the number of individuals per hatch is known to be about 100–300, and the number of individuals per hatch of H. kuda was found to be 70. In the case of seahorses giving birth to a relatively small number of individuals, H. zosterae and Hippocampus give birth to 69 (Strawn, 1958) and 38, respectively, which are more or similar, indicating that the size of the nursery pouch will be an important factor in determining whether the number of bearing larva and juveniles is limited (Masonjones, 1997).
When compared with the size immediately after bearing with seahorse belonging to the same genus, the larva and juveniles immediately after bearing of H. kuda were 6.97–8.81 mm (mean 7.12 mm, n=10), the SL of H. barbouri was 8.82–10.3 mm, H. kuda was 6.66–8.01 mm, 7.0 mm (Mi et al., 1998), H. fuscus was 7.50 mm, H. whitei 8.50 mm (Vincent, 1990), the SL of Hippocampus was 11.6–15.8 mm, H. subelongatus 11.3 mm (Payne & Rippingale, 2000), H. abdomimalis 20.0 mm (Young, 1926), which were larger than or equal to that of H. barbouri and larger than H. kuda but smaller than Hippocampus, showing differences (Table 1).
| Species | Newly bearing of larvae size, mm (mean) | Dorsal fin rays | Pectoral fin rays | Rings trunk+tail | Authors |
|---|---|---|---|---|---|
| Hippocampus kuda | 6.97–8.81 (7.12) | 15–18 | 13–14 | 11+33–36 | Present study |
| 7.00 | 17–18 | 15–18 | 11+34–38 | Lourie et al. (1999b) | |
| 6.66-8.01 (7.59) | 16–17 | 10 | 11+36 | Koh et al. (2004) | |
| H. coronatus | 11.6–15.8 (13.6) | 14 | 12 | 10+39 | Choi et al. 2006b |
| - | 14 | 12 | 10+38–40 | Lourie et al. (1999b) | |
| H. barbouri | 8.82–10.3 (9.48) | 17 | 14 | 11+35 | Choi et al. (2006a) |
| H. histrix | - | 15–18 | 17–20 | 11+33–34 | Lourie et al. (1999b) |
| H. japonicus | - | 16–17 | 12 | 11+39 | Lourie et al. (1999b) |
| H. trimaculatus | - | 18–22 | 16–19 | 11+38–43 | Lourie et al. (1999b) |
| H. fuscus | 7.50 | - | - | - | Vincent (1990) |
| H. whitei | 8.50 | - | - | - | Vincent (1990) |
| H. subelongatus | 11.3 | - | - | - | Payne & Rippingale (2000) |
| H. abdomimalis | 20.0 | - | - | - | Young (1926) |
The number of dorsal fin rays immediately after bearing of H. kuda was 15–18 and that of pectoral fins was 8. Table 1 shows the results of the comparison with Seahorse belonging to the same genus.
In the case of larva and juveniles immediately after bearing of seahorse, each fin and body's bony plate ring have grown close to integer (Mi et al., 1998), and the number of dorsal fin rays, the number and shape of body's bony plate ring for interspecies classification are very useful as the classification traits (Okiyama, 1988; Kanou & Kohno, 2001). On the 12 days after bearing, the number of body's bony plate ring of the top of rings trunk were 11, on the tail of them were 33–36 in H. kuda, seahorse belonging to the same genus was similar, 11 and 36, and Hippocampus had 10, 38–40 (Lourie et al., 1999b), 10, 39 (Choi et al., 2006b), showing that the number of body's bony plate ring on the trunk was more than that on the tail. H. histrix had 11, 33–34 (Lourie et al., 1999b), H. mohnikei had 11, 39 (Lourie et al., 1999b), H. trimaculatus had 11, 38–43 (Lourie et al., 1999b), and the number of body's bony plate ring on the trunk was the same, and the number of body's bony plate ring on the tail was similar or smaller (Table 1).
To summarize the results of this study, H. kuda was identified as a species that give birth to as few as 70 individuals among seahorses and showed the difference from a closely related species in the size of bearing larva and juveniles and the number of body's bony plate ring and thus, we could obtain basic data for species identification.
The dorsal fin basin is described by Lourie et al. (1999a) reported 17–18, but the number of domestic H. kuda and philippines H. kuda were 15–18 and 16–17, respectively. The number of the body was the same as that of the Philippines, and the pectoral fins were 13–14 in domestic and 10 in the Philippines. This result seems to be different according to the distribution, and taxonomic studies will be needed in the near future.
Recently, as the demand for seahorse as an ornamental organism and the demand for a herbal medicine material have increased in Asia, a rapid decrease in resources is expected, and its value is gradually increasing as protective rearing of fish resources in each country. Therefore, it is necessary not only to acquire the basic data of early breeding for artificial breeding and breeding technology of Seahorse, but also to study the early life history for habitat protection and species preservation.

