INTRODUCTION
Fish in the Serranidae family have a market value of more than 3 trillion won worldwide and are consumed at high prices in regions of China. In addition, they are mainly cultured in Asia (Kohno et al., 1993; Harikrishnan et al., 2012). Among them, the red spotted grouper, Epinephelus akaara, is distributed in China, Taiwan, southeast Asia, and the southern and central regions of Japan, much of which is where sub-tropical and tropical rocks and coral reefs are located. In Korea, they inhabit Jeju Island in the south and are in Sori-do and Yokji-do in the north, as well as in some southern coastal areas, especially the Geomundo shore region, which is the center of distribution (Kohno et al., 1993; Harikrishnan et al., 2012). Studies on red spotted grouper have investigated spawning habits, the early life cycle (Ukawa & Higuchi, 1966), gonad development (Hwang et al., 1988), and sperm cryopreservation (Qiutao et al., 2011). In Korea, there have been many studies on red spotted grouper: hepatocyte nuclear changes in fish larvae (Lee et al., 1998), growth hormone expression (Kang et al., 2003), maturation and sexual reversal (Lee et al., 1998), live food (Lee & Hur, 1997), egg quality changes (Lee et al., 1997), egg and fish larvae development (Park et al., 2016), and hybrid embryo hatching (Noh et al., 2015). Despite these various studies, there have been few studies on seed production, including technology for securing high-quality embryos and early live foods of the red spotted grouper, which are required in the fishery industry.
One of the most important factors in fishery production is fish seeds with which to secure large amounts of embryos at the same time. However, similar to most of the Serranidae family, red spotted groupers are hermaphroditic fish that undergo protogynous dichogamy and sexual reversal into males when they reach approximately 30 cm in total length. Therefore, because the frequency of mature males among red spotted grouper caught in nature is very low, obtaining males for securing the necessary sperm for the production of artificial seeds has been recognized as an important issue in seedling production. Thus, the importance of effective management of spawning broodstock has been highlighted. In addition, species-specific reproductive and ecological characteristics make it difficult to control the breeding environment for natural spawning (Toledo et al., 1993; Okumura et al., 2002). In general, embryos of fish, including those in the Serranidae family, are difficult to naturally spawn and rather are produced through hormone treatment (Chen, 1990; Tucker et al., 1994). For this reason, most of the Serranidae family fish embryos are produced through natural spawning induced by hormone treatment after identifying the size of oocytes through cannulation. The embryos are also produced through egg collection by pressing the abdomen of the fish. In Korea, the mother fish are managed in cage cultures or indoor tank cultures for mass production of red spotted grouper seeds, and the mother fish are screened during the spawning season, and embryos are produced by pressing the abdomen after hormone treatment. However, mass production of embryos in the industry requires a great deal of time and labor to identify the maturity of each egg by cannulation. When cannulation is repeatedly performed to determine the maturity of the eggs, the egg quality may be lowered because of the stress on the mother (Cho et al., 2016). In addition, the standard for identifying the maturation period visually without cannulation is unclear. Thus, hormones may be given too early, or the correct hormone treatment time may pass. Therefore, there is a problem in the production of high-quality embryos in large quantities.
Therefore, this study was conducted to establish the hormone treatment time and screening criteria for target mother fish for seed production based on the external form of a red spotted grouper mother candidate group. This study also aimed to provide a framework for stable production of high-quality embryos by easily applying these criteria in mass seed production in the industry. The degree of abdomen inflation of the mother fish based on the anal fin was classified, gonadosomatic index (GSI) was determined, and ovaries and eggs were observed according to the degree of abdomen inflation (Miura et al., 2014). Fertilization rate, floatation rate, embryonic survival rate, and hatching rate based on the degree of abdomen inflation were examined, the possibility of screening mothers based on external forms was confirmed, and the quality of eggs was compared.
MATERIALS AND METHODS
Three-hundred forty-three red spotted groupers (38.9± 4.1 cm in total length, 885.3±143.5 g in weight) were caught in along the Geomun-do shore region in Yeosu, Jeollanam-do in June 2013 and cultured in a cage (5×5×5 m). In June 2014, mature mother fish were selected and treated with hormones according to the degree of abdomen inflation based on weight and the anal fin of the mother fish (Fig. 1).
LHRHa (des-Gly10, D-Ala6; Sigma Co. LLC., USA) was used as a hormone for egg maturation and induction of ovulation. It was injected into the lower back muscle at the first spinous ray at a concentration of 100 µg/kg (Liao & Leano, 2008). The fish were separated into a small cage (2×2×2 m) for each experimental group.
Forty-eight hours after hormone injection, the abdomen of the test fish was pressured to examine ovulation and the amount of eggs collected per mother fish. Ten collected embryos were observed under a stereoscopic microscope (Olympus CX41, Japan) to measure egg diameter and oil globule diameter. The eggs and semen were artificially fertilized using the dry method, and the fertilization rate and floatation rate were examined. The embryonic survival rate until hatching and hatching rate 12 hours after hatching were determined.
To determine the minimum body weight required for egg maturation and ovulation induction, 40 female fish (32.5±1.7 to 39.9±2.0 cm in total length, 550.7±65.5 to 1,110.0±65.8 g in body weight) were divided into four weight groups (400–600, 600–800, 800–1,000, and 1,000–1,200 g). Estimated age was 5–6 (400–600 g), 6–7 (600–800 g), 7–8 (800–1,000 g) and over 8 (1,000–1,200 g) years old oocytes (Lee & Lee, 1996). Then, 10 fish were selected from each group and treated with the hormone. Next, the frequency of spawning, amount of eggs collected per mother fish, fertilization rate, floatation rate, embryonic survival rate, and hatching rate were examined following spawning after hormone treatment (Table 1).
| Variables | 400–600 | 600–800 | 800–1,000 | 1,000–1,200 |
|---|---|---|---|---|
| TL (cm) | 32.5±1.7 | 35.6±1.5 | 37.6±1.7 | 39.9±2.0 |
| BW (kg) | 550.7±65.5 | 722.1±57.0 | 905.1±46.7 | 1,110.0±65.8 |
TL, total length; BW, body weight.
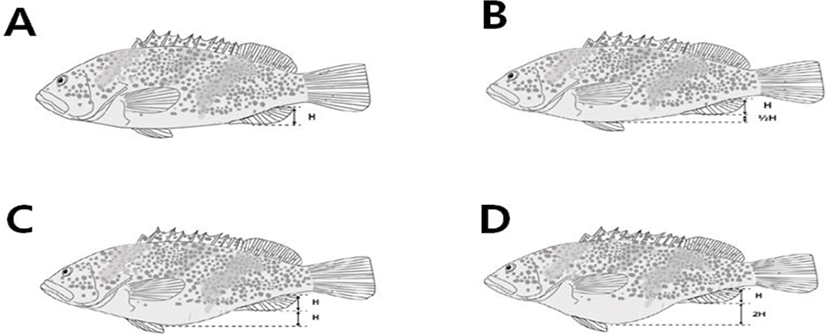
To establish the criteria for screening mother fish and hormone treatment time based on external forms, 60 mature females (32.6±4.0 to 37.7±2.7 cm in total length, 9.3±0.8 to 14.2±1.1 cm in body length, 561.4±5.4 to 972.2± 3.8 g in body weight) were classified by the degree of abdomen inflation into I–IV stages based on the anal fin (Fig. 2, Table 2). Ten mother fish were selected for each stage according to the criteria and treated with the hormone. The amount of eggs collected per mother fish, fertilization rate, floatation rate, embryonic survival rate, and hatching rate were investigated according to stage. Five mother fish were dissected to measure gonad weight and calculate GSI.
| Variables | Stage | |||
|---|---|---|---|---|
| I | II | III | IV | |
| TL (cm) | 32.6±4.0 | 33.5±4.9 | 36.9±3.7 | 37.7±2.7 |
| BD (cm) | 9.3±0.8 | 10.4±0.8 | 12.5±1.1 | 14.2±1.1 |
| BW (kg) | 561.4±5.4 | 753.4±2.7 | 912.5±2.5 | 972.2±3.8 |
Each value represents mean±SD (n=10 for each stage). TL, total length; BD, body depth; BW, body weight. Stage Ⅰ, parallel to the bottom of anal fin and abdomen; Stage Ⅱ, height of the anal fin was twice height of the abdomen inflation; Stage Ⅲ, height of the anal fin and abdomen inflation were the same; Stage Ⅳ, height of the abdomen inflation was twice height of the anal fin.
The GSI was calculated using the following equation:
To histologically observe gonadal development at each stage, ovaries fixed in 10% neutral formalin were re-fixed with Bouin's solution. The fixed tissues were prepared into blocks by the paraffin embedding method. Then, the samples were serially sectioned into 4 µm sections by the paraffin sectioning method. Next, the samples were counterstained with Mayer's hematoxylin-eosin (H–E) and observed under an optical microscope (Olympus, CX31) equipped with an image measurement system (FOCUS technology, 2005).
RESULTS
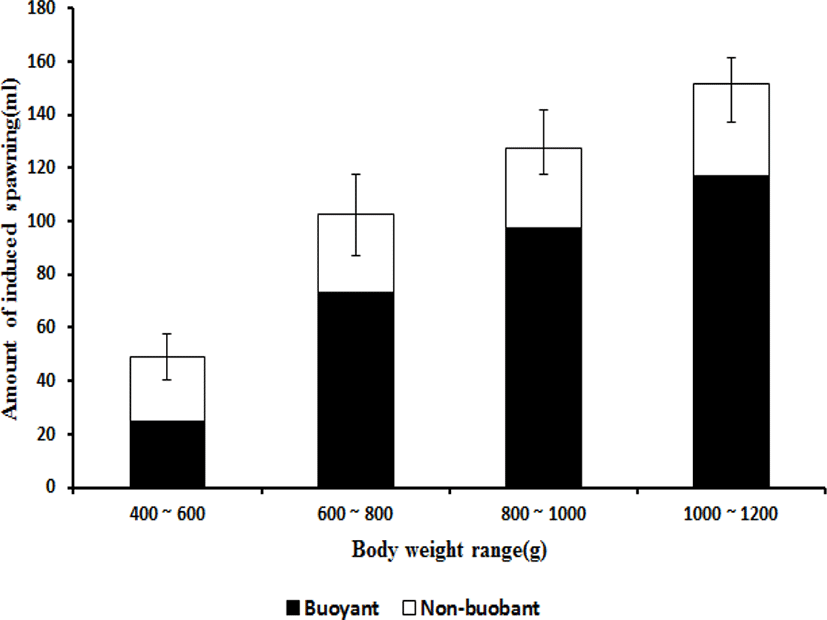
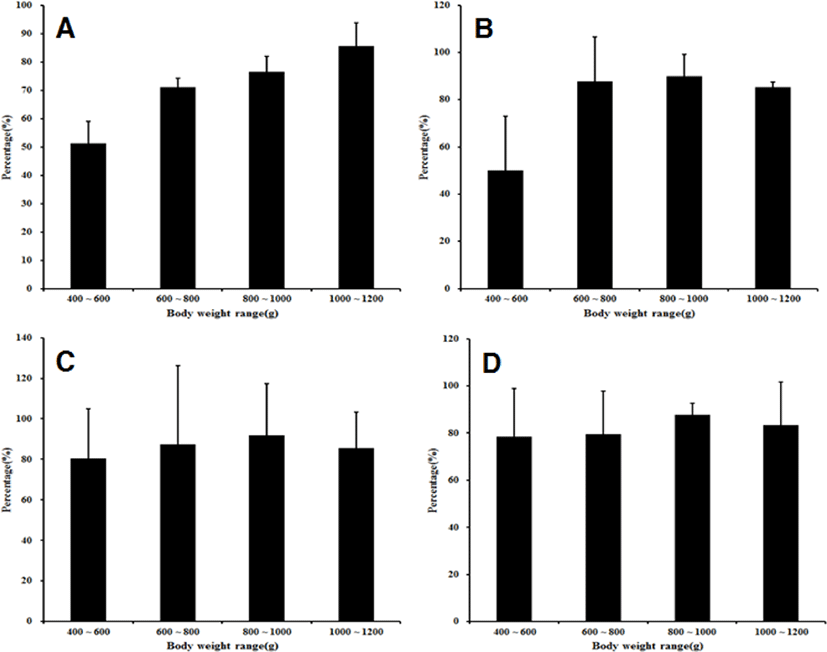
As a result of examining the ovulation induction effect on the mother candidate group managed in the cages according to body weight, all of the mother fish with a body weight of 600 g or more artificially spawned eggs, whereas only 70% of the mother fish with a body weight of 400–600 g spawned eggs. In the range of 400–600 g, seven mother fish spawned, and the amount of eggs collected per mother was 48.8±9.9 mL of which 24.6±3.5 mL were floating eggs. In the range of 600–800 g, all 10 mother fish spawned, and the amount of eggs collected per mother was 102.4±10.4 mL, of which 73.0±18.2 mL were floating eggs. In the range of 800–1,000 g, all 10 mother fish spawned, and the amount of eggs collected per mother was 127.4± 16.5 mL, of which 97.2±14.6 mL were floating eggs. In the range of 1,000–1,200 g, all 10 mother fish spawned, and the amount of eggs collected per mother was 151.8± 12.9 mL, of which 117.0±14.5 mL were floating eggs (Fig. 3). The flotation rate was highest (85.5±8.2%) in the 1,000–1,200 g range, and it was 76.6±5.3% in the 8,00–1,000 g range. It was 71.0±3.2% in the 600–800 g range, and it was lowest (51.2±7.8%) in the 400–600 g range. The highest fertilization rate was 89.8±9.2% in the 800–1,000 g range, and the fertilization rate was 87.8±18.6% in the 600–800 g range. The fertilization rate was 85.4±2.16% in the 1,000–1,200 g range, and it was lowest (50.2±22.8%) in the 400–600 g range. The embryonic survival rate was highest at 91.8±25.3% in the 800–1,000 g range, and it was 87.5±38.6% in the 600–800 g range. It was 85.6± 17.5% in the 1,000–1,200 g range and 80.4±24.5% (lowest) in the 400–600 g range. The hatching rate was the highest (87.8±4.9%) in the 800–1,000 g range. It was 83.5±18.2% in the 1,000–1,200 g range, 79.5±18.4% in the 600–800 g range, and 78.5±20.5% (lowest) in the 400–600 g range (Fig. 4).
To establish the criteria for determining the hormone treatment time by the naked eye, the degree of abdomen inflation was divided into I–IV stages based on the anal fin. As a result, at stage I, all mother fish showed normal spawning, but a small number of eggs appeared, and many of them were immature. The average amount of collected eggs per mother fish was small with a value of 9.0±2.0 mL, of which 3.8±2.1 mL was of floating eggs. At stage II, most of the eggs were mature, and the amount of eggs collected per mother fish was 20.4±8.3 mL, of which 9.6±4.3 mL was of floating eggs. At stage III, most of eggs were mature, and the amount of eggs collected per mother fish was 137.4±18.1 mL, of which 112.6±8.4 mL was of floating eggs. At stage IV, most eggs were over-mature, and the amount of eggs collected per mother fish was 150.2±16.2 mL, of which 93.6±26.4 mL was of floating eggs (Fig. 5).
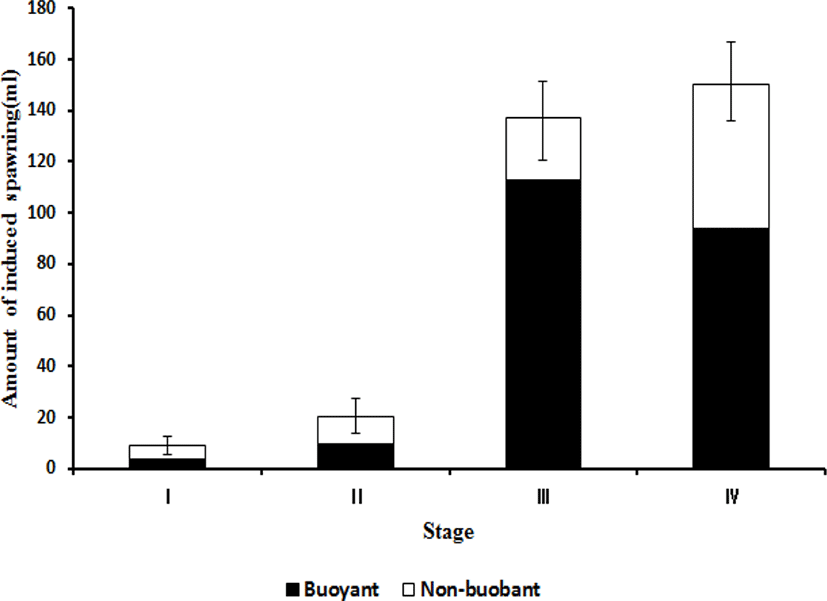
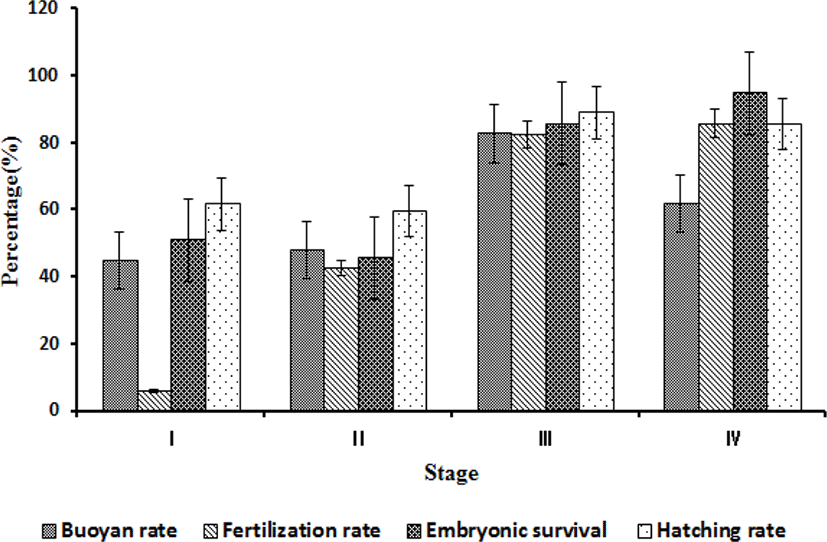
The flotation rate was highest (82.6±8.6%) at stage III, followed by 61.6±12.4% at stage IV, 47.7±19.0% at stage II, and 44.7±29.1% at stage I. The fertilization rate was highest (85.5±2.16%) at stage IV, followed by 82.4±9.2% at stage III, 42.5±18.6% at stage II, and 5.8±8.1% at stage I. The embryonic survival rate was highest (94.5±17.5%) at stage IV, followed by 85.4±25.3% at stage III, 50.8± 24.5% at stage I, and 45.5±38.6% at stage II. The hatching rate was highest (88.8±4.9%) at stage III, followed by 85.5±18.2% at stage IV, 61.5±20.5% at stage I, and 59.5±44.4% at stage II (Fig. 6).
The egg characteristics (egg diameter and oil globule diameter) and GSI were measured to investigate the sexual maturity stage according to the degree of abdomen inflation. As a result, at stage I, the egg diameter was 0.76±0.01 mm (0.74–0.77 mm), and oil globule diameter was 0.17± 0.01 mm (0.16–0.19 mm). At stage II, the egg diameter was 0.75±0.02 mm (0.74–0.80 mm), and oil globule diameter was 0.16±0.01 mm (0.15–0.17 mm). At stage III, the egg diameter was 0.75±0.01 mm (0.73–0.78 mm), and the oil globule diameter was 0.17±0.01 mm (0.16–0.17 mm). At stage IV, the egg diameter was 0.73±0.02 mm (0.72–0.75 mm), and the oil globule diameter was 0.16±0.01 mm (0.16–0.17 mm). Thus, the egg diameter was highest at stage I and lowest at stage IV. There was no significant difference in oil globule diameter based to stage (Table 3).
| Variables | Eggs (mm) | Oil globules (mm) |
|---|---|---|
| Stage I | 0.76±0.01 | 0.17±0.01 |
| Stage II | 0.75±0.02 | 0.16±0.01 |
| Stage III | 0.75±0.01 | 0.17±0.01 |
| Stage IV | 0.73±0.02 | 0.16±0.01 |
Each value represents mean±S.D. (n=10 for each female). Stage Ⅰ, parallel to the bottom of anal fin and abdomen; Stage Ⅱ, height of the anal fin was twice height of the abdomen inflation; Stage Ⅲ, height of the anal fin and abdomen inflation were the same; Stage Ⅳ, height of the abdomen inflation was twice height of the anal fin.
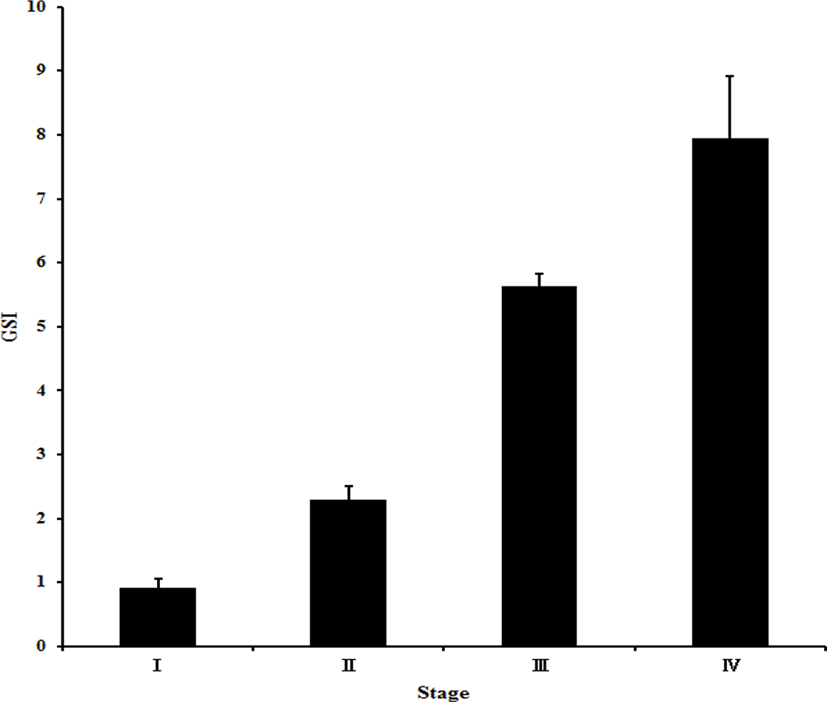
The GSI value was 0.9±0.2% at stage I and 2.3±0.2% at stage II. The GSI value at stage III was 5.6±0.2%. Lastly, stage IV showed a GSI value of 7.9±0.9% (Fig. 7).
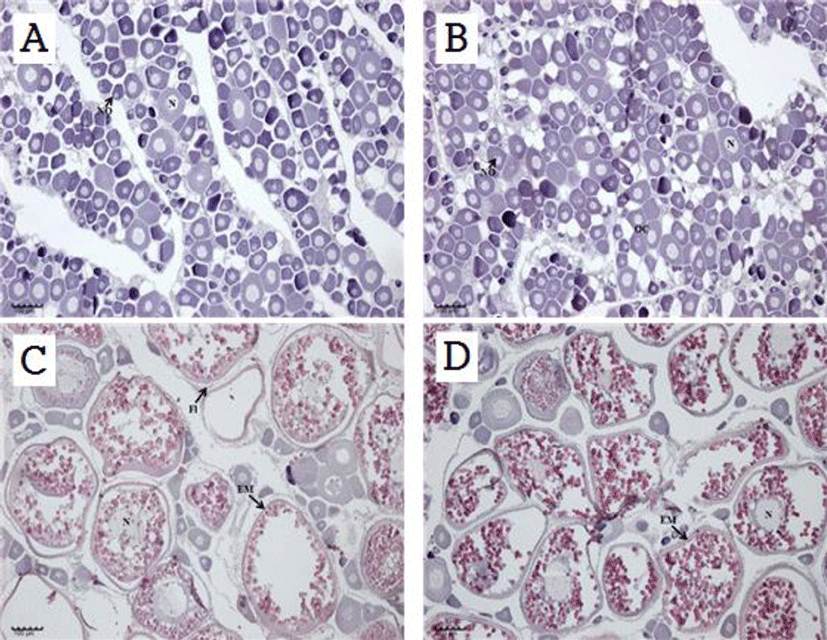
Histological examination of the gonads revealed that stage I and stage II consisted mostly of early oocytes and nuclei occupied a high proportion of the cytoplasm (Fig. 8A and B). At stage III, most eggs were mature eggs with accumulated yolk, and at stage IV, almost all eggs were mature (Fig. 8C and D).
DISCUSSION
In general, fish become mature and spawn at a specific season, exhibiting a reproductive cycle around the spawning season. Water temperature and photoperiod have been reported to be environmental factors controlling the reproductive cycle (De Vlaming, 1972, De Vlaming, 1975; Lee et al., 1984). According to the spawning time, fish were classified into six types: spring, spring-summer, spring-fall, summer, fall, and winter (Aida, 1991). It was considered that gonad maturation of red spotted groupers, which belong to the summer spawning type, was influenced by the increase in temperature and long photoperiod. In addition, when the oocytes of the fish were classified into synchronous, group-synchronous, and asynchronous based to the development of oocytes (Wallace & Selman, 1981; De Vlaming, 1983), each mother fish spawned normal eggs, immature eggs, or over-mature eggs simultaneously. Red spotted groupers, which have oocytes during the yolk vesicle and yolk globule stages in the mature ovary simultaneously belong to the asynchronous type, which spawns at multiple times.
At weight range 400–600 g group, collected eggs was the least and most of the eggs were immature. It is supposed to be more than 600 g in order to produce mature eggs stably. It is considered that size of the mother fish affects the maturation of eggs.
The size of oocytes was measured through cannulation during the spawning season for seed production to determine hormone treatment time. In this study, we aimed to verify the applicability of determining hormone treatment time through the degree of abdomen inflation measured by the naked eye instead of determining hormone treatment time by measuring the size of oocytes through existing cannulation for seed production of E. septemfasciatus. As a result, the quality of eggs differed according to the degree of abdomen inflation of the mother fish in terms of the amount of eggs collected per mother fish, maturation degree, and histology. At stage III, where the abdomen inflation degree of the mother fish was 1 based on the basal part of the dorsal fin relative to the height of the anal fin, the egg quality was the highest. At stage I with the degree of abdomen inflation of 0, the amount of eggs collected per mother fish was the least and most of the eggs were immature. At stage II where the degree of abdomen inflation was 1/2, most of eggs were mature, but the amount of eggs per mother fish was small. At stage III, eggs were mature with accumulated yolk, the flotation rate was highest with a value of 82.6±8.6%, and the hatching rate was highest with a value of 88.8±4.9%. At stage IV, the amount of eggs collected per mother fish was greatest; however, most of the eggs were mature histologically, and over-mature eggs were observed. It was necessary to study the specific time so that ovulation induction is more stable by subdividing maturity stages III and IV.
Temperate bony fish that spawn in a certain season undergo body changes according to gonad development during the spawning season. The GSI has been most widely used to identify the spawning season. The GIS at stage III, which showed the highest egg quality of red spotted grouper, was 5.6±0.2%, which was significantly different from the highest value (2.42%) of Hwang et al. (1998). The highest value of 7.69% in Lee et al. (1998) was similar to the GSI at stave IV in this study. In addition, the GSI was higher than 4.68% of the Serranidae family E. septemfasciatus (Cho et al., 2016). Compared with other Perciformes fish, the results of our study were higher than the 4.47% in Micale et al. (1996) for Diplodus puntazzo, 0.06%– 1.86% in Papadaki et al. (2008), and 1.14% in Hernandez et al. (2003). However, the results in this study were lower than the 8.21% of Perca fluviatilis (Abdulfatah et al., 2013), 10.8% of mandarin fish (Lee et al., 2012), 12.85% of dragonet (Baeck & Huh, 2004), and 7.50% of spotted sea bass (Kim et al., 2001). These differences may have resulted from the GSI being affected by differences in the age and size of mother fish caught in nature.
In conclusion, the results of this study suggest that hormone treatment time and mother screening criteria can be determined by visual methods, such as the degree of abdomen inflation, for seed production of red spotted grouper. If visual indicators that can be used as criteria for determining hormone treatment time and screening of mother fish are identified in addition to the degree of abdomen inflation, it would be possible to easily determine hormone treatment time and screen mother fish without causing stress to the mother fish, as it would not require cannulation. Thus, such criteria could be applied in the industry.