INTRODUCTION
Water temperature is one of the vital environmental variables that influence the biology of fishes (Dunham et al., 2003). The rise in water temperature due to global warming is a great concern of aquaculturists and fishery biologists. The fluctuations in water temperature may significantly provoke the normal physiological processes, growth and survival of teleost fish, thereby declining the fish abundance (Portner & Peck, 2010). The normal water temperature range in the tropics to which fish are well adapted is 25°C–35°C (Howerton, 2001). Although the thermal preference level varies from species to species, however, temperature can rise to a point that could be harmful for fish growth and impairment of physiological processes (Fu et al., 2018).
Blood is the most efficient indicator related to immune response that express endogenous or exogenous changes in fish (Bowden et al., 2007; Simide et al., 2016). It is easy to collect fish blood and the parameters associated with blood can provide sufficient information about the immune system of fish to environmental changes that affect homeostasis (Cazenave et al., 2005; Elahee & Bhagwant, 2007). The morphology and number of erythrocytes vary with ambient temperature (Ytrestoyl et al., 2001). It has been reported that various erythrocytic alterations due to thermal stress are effective indicator of cytotoxicity (Shahjahan et al., 2018; Islam et al., 2019). Cell shrinkage and membrane blebbing are signs for apoptotic cells (Talapatra & Banerjee, 2007). Though a number of studies have been conducted for better understanding of physiological thermal stress responses of aquatic organisms (Ahmad et al., 2011; Cheng et al., 2013), the high temperature effects on blood profiles of red spotted grouper Epinephelus akaara, are not well studied. Analysis of blood profiles in response to thermal exposure is required to get a rapid feedback about the immune response of the stressed fish and thus will help to develop advance management strategies for the improvement of fish habitat in consideration of rising sea water temperature.
Groupers are warm water fish that spawn and grow best at 24°C to 30°C; most of them prefer a thermal range of 15°C to 35°C (Tucker, 1999). Among the groupers, the red spotted grouper, E. akaara is a sub-tropical species that occurs in southern Japan, Korea, Hong Kong, Taiwan, and southern China (Kim et al., 2005). It is a commercially important species in southeastern Asia due to its high market value (Morris et al., 2000). However, overexploitation, habitat degradation, and global climate change effects have reduced the landings of this species, making natural stocks vulnerable (Tupper & Sheriff, 2008). The International Union for the Conservation of Nature (IUCN) has listed E. akaara as an endangered species (Sadovy et al., 2018). Therefore, appropriate management and conservation measures are necessary to protect this endangered species.
Nowadays, it has been observed that the seawater temperature exceeds beyond the normal tolerance range of fish (24°C to 30°C) (Noyes et al., 2009). Considering this, we have investigated the thermal effects on red spotted grouper in our previous studies and shown that high thermal exposure (>30°C) can alter the hematological and biochemical parameters [Cho et al., 2015; Lee & Baek, 2018; Rahman et al., 2019 (unpublished data)]. Nonetheless, there is no published information about the blood characteristics of red spotted grouper (E. akaara) at higher thermal exposure. Therefore, in the present study, we intended to know how high water temperature affects the blood cell morphology and number in red spotted grouper after rearing at longer periods.
MATERIALS AND METHODS
Healthy and active juveniles of E. akaara were obtained from the Marine Science Institute, Jeju National University, Korea. The mean total length (TL) and body weight (BW) were 8.28±0.10 cm and 8.53±0.27 g, respectively. Fish were acclimated in aquaria at a water temperature of 25±0.5°C, salinity of 34 psu, dissolved oxygen levels≥6.6 mg/L, pH of 7.8, and a 12L:12D photoperiod for 2 weeks before the experiment. Fish were fed commercial feed (Otohime Hirame; Marubeni Nisshin Feed Co., Ltd., Tokyo, Japan) twice daily at 09:00 and 18:00 h until satiation, until 24 h before the experimental trial. Uneaten food was removed after 30 minutes of feeding to control water quality.
A total of 180 juvenile red spotted grouper were selected and randomly distributed into 12 rectangular glass tanks (75×45×45 cm, W×L×H) containing 15 fish per tank. Each tank was filled with 120 L seawater and equipped with a recirculating filtration system. The fishes were exposed to four temperature conditions (25°C as control, 28°C, 31°C, and 34°C), each with three replications for 6 weeks. Temperature was gradually increased (Δ1°C/h) using a thermostat (OKE-6422H; OKE, Busan, Korea) to reach the target temperature conditions (25°C, 28°C, 31°C, and 34°C). Each experimental aquarium was maintained at a constant temperature±0.5°C with supplemental aeration and skimming. Survival of the experimental fish was monitored over the entire rearing period. Water temperature, pH, salinity, and dissolved oxygen were checked daily using a water quality meter (HI9829; Hanna Instrumentals, Woonsocket, RI, USA) and total ammonium levels were measured every 2 days using an NH3/NH4+ test kit (Tetra GmbH, Melle, Germany). Approximately 10% of the water was replaced daily with filtered clean seawater as feces and debris were siphoned from the tank. Fish maintenance, handling, and sampling were conducted according to the guidelines of the Animal Ethics Committee of Pukyong National University (PKNU) (Regulation No. 554).
Fish were sampled at 2 days, 1 week, and 6 weeks after the thermal exposure starting point. At each sampling point, five fishes were sampled from each aquaria (15 fishes from each temperature group; n=15). Fish were randomly captured and lightly anaesthetized with 300 ppm 2-phenoxyethanol (Sigma Aldrich, St. Louis, MO, USA). Blood was collected from the caudal vein using heparinized capillary tubes, labeled, and stored in 1.5 mL centrifuge tubes for analysis of blood indices. Whole blood withdrawal process was done less than 1 min per fish to avoid handling stress.
The red and white blood cells (RBC and WBC) were counted under a light microscope (BX-50; Olympus, Tokyo, Japan) using an improved Neubauer Hemocytometer (Blaxhall & Daisley, 1973). Blood was smeared on clean microscopic slides immediately after collection, air-dried for 10 min, fixed in methanol for 10 min, stained with Wright-Giemsa solution, rinsed with distilled water, air-dried and mounted with malinol. Three blood smeared slides were prepared from of each fish and 100 cells were scored from each slide. Frequencies of differential leucocytes were recorded from blood smears according to the methodology proposed by Davis et al. (2008).
Erythrocytic cellular abnormalities (ECA) and differential leucocytes, such as monocytes (Mo), neutrophil (N), lymphocytes (L), eosinophil (Eo), and basophil (Ba) were examined from the blood smear of fishes reared in different thermal conditions under a light microscope (BX-50; Olympus, Tokyo, Japan; ×600 magnification). The ECA were dissimilar from the normal erythrocyte cell (oval-shaped with a condensed nucleus).
All data are presented as means±SEM. One-way analysis of variance (ANOVA) followed by Duncan's multiple range test was used to assess the significant differences among the different temperature groups. Statistical significance was set at p<0.05. Data analysis were performed using SPSS statistics software (ver. 21.0; IBM Corp., Armonk, NY, USA).
RESULTS
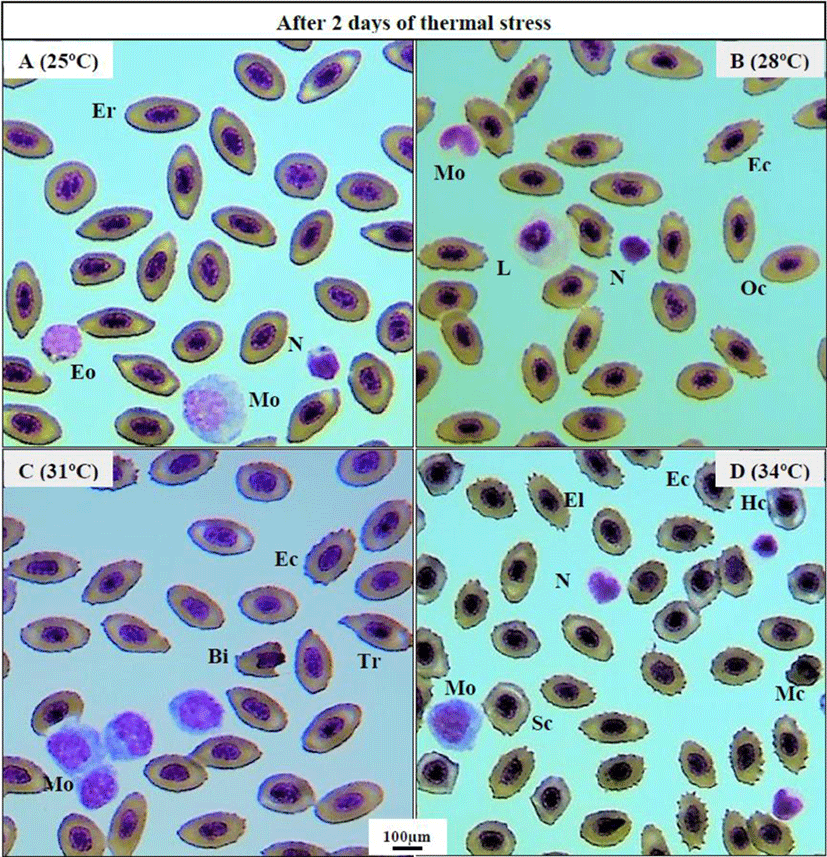
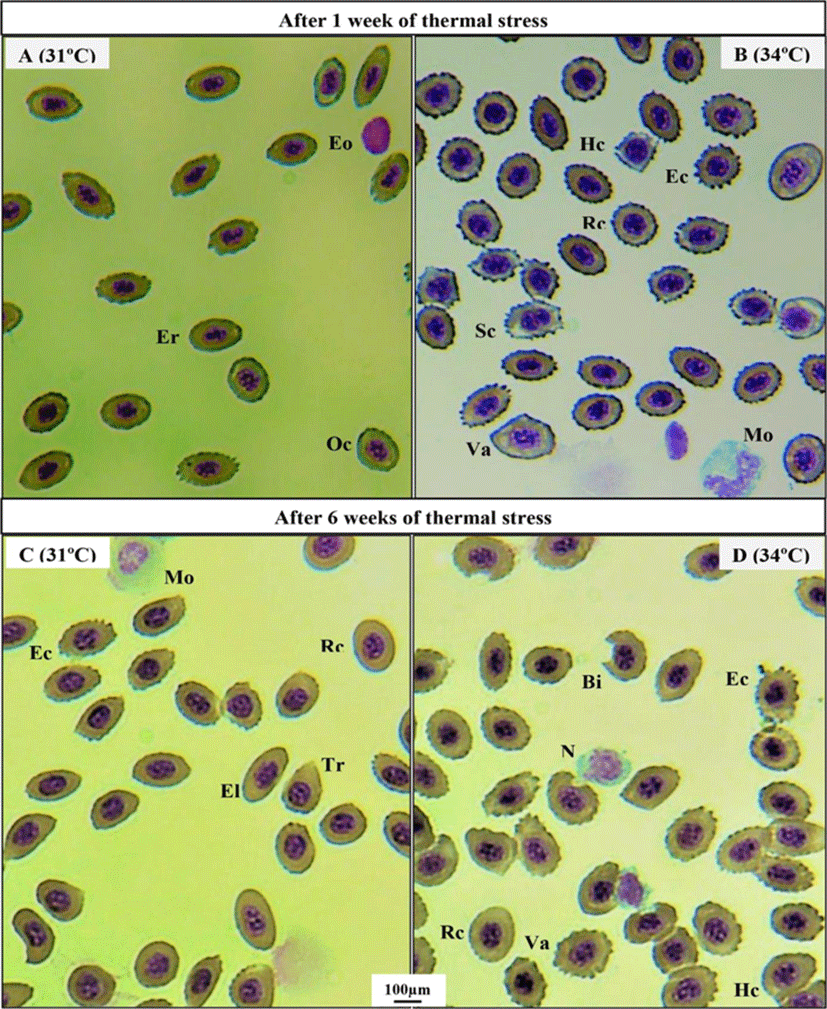
Thermal exposure induced various ECAs in 31°C and 34°C groups on day 2 (Fig. 1C, D) compared to those in the 25°C and 28°C groups (Fig. 1A, B). After 1 week, 34°C induced more erythrocytic damage showing echinocytes (Ec) and swelled blood cells (Sc) (Fig. 2B), and 31°C group showed normal erythrocyte structure (Er) with oval shaped (Oc) (Fig. 2A). However, 25°C and 28°C group did not show any erythrocytic abnormalities after 1 and 6 weeks of heat exposure (Figure not shown). After 6 weeks, massive alteration in erythrocytes continued in 34°C group (Fig. 2D) and a sign of erythrocytic (Er) stress was also recorded in 31°C group (Fig. 2C). The other major signs of RBCs alterations included tear drop like cells (Tr), eliptocyte cells (El), round shaped cells (Rc), microcytic RBCs (Mc), bite cell (Bi), hemolyzed RBC cells (Hc), and vacuolated cells (Va).
Thermal exposure significantly elevated the RBCs at 34°C on day 2, whereas WBCs increased at 31°C and 34°C group. After 1 week, both RBC and WBC number were significantly elevated at 34°C compared to those in the 25°C, 28°C, and 31°C groups. However, after 6 weeks, thermal stress significantly raised both RBC and WBC number in 31°C and 34°C group (Table 1).
Values are mean±SEM of three replicates (n=15; 5 fish per tank).
Different lowercase letters in each column indicate the significant difference between groups at equivalent times (ANOVA, Duncan's multiple range test; p<0.05).
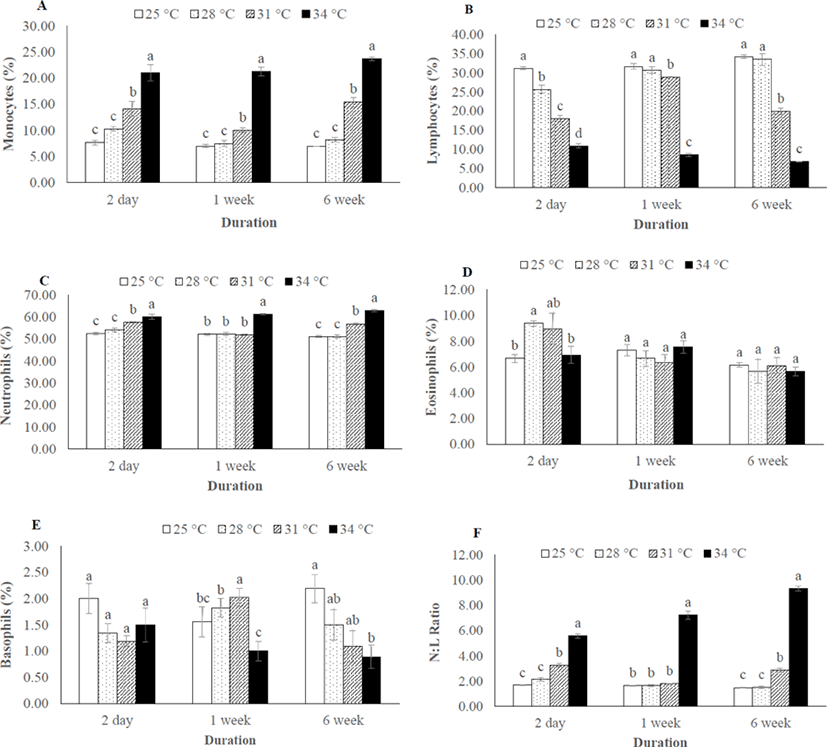
Monocyte and neutrophil number were significantly higher in 31°C and 34°C throughout the thermal exposure period (Fig. 3A, C), whereas the number of lymphocytes were decreased in 31°C and 34°C (Fig. 3B). As consequences, the N:L ratio was significantly different among the thermal exposure groups (Fig. 3F). However, no change was recorded in eosinophil and basophil number in any group after 6 weeks of thermal exposure (Fig. 3D, E).
DISCUSSION
Temperature beyond the tolerance range for a particular species adversely affects its immune capacity, hence increase the susceptibility to diseases that have suppressive effects on growth and reproduction (Cnaani, 2006). In this study, red spotted grouper juveniles were overwhelmed by thermal stress at higher exposure (31°C and 34°C), which affects their blood cell morphology and number. Fishes of 34°C were severely affected after 6 weeks of thermal exposure and showed less appetite for feed, gulping for air and irregular body movement. The common carp (Cyprinus carpio var. communis) exhibited similar behavior when exposed to 32°C for a period of one month (Ahmad et al., 2011).
In the present study, several ECA like echinocytes, teardrop-like cells, swollen cells and vacuolated cells were observed in blood smears of thermally exposed fish (31°C and 34°C) in comparison with other groups (25°C and 28°C). These results clearly indicate the sign of stress which can lead to an anemic condition of experimental animals at higher thermal exposure (34°C). Similar erythrocytic alterations were reported in striped catfish, Pangasianodon hypophthalmus when treated with higher temperature (36°C) for 7 and 28 days (Islam et al., 2019). The observed vacuoles in erythrocytes may be resulted from unequal distribution of hemoglobin (Mekkawy et al., 2011). The swelled blood cells were recorded as signs of necrosis (Ateeq et al., 2002). Echinocytes is type of erythrocytic abnormality that formed due to interruption in lipid solubility to erythrocytes membrane which ultimately leads to apoptosis (Walia et al., 2013).
In this study, the elevated RBCs at 31°C and 34°C after 6 weeks thermal exposure indicates that the stressed fish tried to cope with adverse conditions by enhancing their respiratory capabilities through evaluated RBC. The higher RBC levels may increase the oxygen carrying capacity of blood, thus supplying oxygen to major organs in response to higher metabolic demand, which is a manifestation of stress (Ruane et al., 1999). Moreover, the observed higher WBC number at 31°C and 34°C after 6 weeks thermal exposure can be correlated with additional antibody demand that may help the fish to survive in adverse environmental condition. Similarly, RBC and WBC number were found to be increased with increased water temperature in striped catfish (24°C to 36°C) and common carp (20°C to 32°C) when reared for a period of one month (Ahmad et al., 2011; Islam et al., 2019).
In the present study, the observed increased neutrophil (N) and decreased lymphocytes (L) among the differential leucocyte number in the higher temperature (31°C and 34°C) indicates the disturbance of immune system which may lead to infectious disease to the thermally stressed fish. Similar results has been reported in P. hypophthalmus where increases neutrophils numbers (neutrophilia) and decreases lymphocyte numbers (lymphocytopenia) due to thermal stress (Shahjahan et al., 2018). Moreover, the N:L ratio can be used as an index of a secondary stress response, since the neutrophils and lymphocytes numbers are affected by stress in opposite directions (Davis et al., 2008). The present study also supports this statement as the highest N:L was recorded in 34°C group. The higher N: L similar to our study was also recorded in juvenile dusky grouper Epinephelus marginatus (Pereira-Cardona et al., 2017) and pejerrey fingerlings Odontesthes bonariensis (Zebral et al., 2015) where stress arouse from transport and crowding, respectively.
In conclusion, the results of the present study demonstrate that thermal stress may affects the immune system of E. akaara at higher temperature (31°C and 34°C) by altering the blood cells morphology and number. In addition, different N:L ratio at different thermal conditions can be used as a reliable alternative to measure stress levels in juvenile E. akaara and probably in other fish species.

