INTRODUCTION
Diabetes mellitus (DM) is a metabolic syndrome that is classified into two types: type 1 DM (T1DM) and type 2 DM (T2DM) (Thomas & Philipson, 2015). While T1DM is linked with failure in insulin production by pancreatic β-cells, T2DM is characterized by insulin resistance. Insulin resistance is generally identified as an impaired response to insulin stimulation of the liver, muscle, and adipose tissue. Insulin resistance causes glucose disposal and subsequently results in a compensatory increase in β-cell insulin production (Deacon, 2019). The metabolic consequences of insulin resistance can cause hyperglycemia, hypertension, dyslipidemia, visceral adiposity, and hyperuricemia (Lebovitz, 2001; Freeman & Pennings, 2020). Thus, progression of insulin resistance can provoke metabolic syndromes, nonalcoholic fatty liver disease (NAFLD), and T2DM. Therefore, most T2DM medications have been developed to enhance insulin sensitivity. At present, metformin, a derivative of galegine (a natural product from the plant Galega officinalis), is the most representative therapeutic drug for T2DM (Sanchez-Rangel & Inzucchi, 2017). Metformin functions by activating one of the enzymes involved in gluconeogenesis, known as 5-adenosine monophosphate-activated protein kinase (AMPK) (Rena et al., 2017).
Silk protein fibroin (SPF) from spiders has been suggested as a useful biomaterial for industrial and medical purposes (Altman et al., 2003; Kluge et al., 2008). The biological effects of SPF include enhancement of glucose and lipid metabolism (Hyun et al., 2004; Jung et al., 2010; Do et al., 2012), anti-viral activity (Gotoh et al., 2000), DNA damage protection (Park et al., 2002), and blood pressure-depressing activity (Igarashi et al., 2006). Recombinant spider SFP exhibits non-cytotoxic and non-inflammatory effects in NIH 3T3 cells (Lee et al., 2016) and reduces blood glucose levels in diabetic mice (Lee et al., 2014; Park et al., 2019). Recently, we demonstrated that dietary exposure to transgenic rice (TR) that expresses spider SPF reduces blood glucose levels, possibly through the potential mediating role of insulin receptor substrated-1 phosphorylation in adipocytes (Park et al., 2019). However, the fundamental mechanism(s) by which TR-SPF lowers blood glucose levels in diabetic mice remains to be elucidated in hepatic tissues.
In the present study, TR-SPF was fed to diabetic BKS.Cg-m+/+Leprdb mice, changes in glucose and insulin levels were monitored in blood, and alterations in proteins associated with insulin signaling and insulin resistance were analyzed in hepatic tissues. The purpose of this study was to determine whether specific proteins related to insulin resistance in hepatocytes correlate with changes in blood glucose levels in TR-SPF-fed mice.
MATERIAlS AND METHODS
Bouin’s solution, eosin and hematoxylin were purchased from Sigma Chemical (St. Louis, MO, USA). The western enhanced chemiluminescence (ECL) detection reagent was purchased from Bio-Rad (Hercules, CA, USA). VECTASTAIN® Elite ABC Kit was from Vector Laboratories (Burlingame, CA, USA). Anti-AMPKα, Phospo-AMPKα (Thr172), Akt and Phosphor-Akt (Ser473) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-STEAP4 (six-transmembrane protein of prostate 2, STAMP2) antibody was from Proteintech (Rosemont, IL, USA). Anti-β-Tubulin antibody was from Sigma Chemical (St. Louis, MO, USA).
cDNAs encoding a partial repetitive region and the C-terminal domain of AvMaSp (Lee et al., 2012) were PCR-amplified from pGEMT-AvMaSp using the forward primer 5’-GGATCCATGGCCGCCGCAGCCGCA-3’ (with BamHI) and reverse primer 5’-GTCGACTAACGCTGCGGCAGAGGC-3’ (with SalI). The AvMaSp fragment was inserted into the binary pPZP-3’PINII-Bar vector (pAvMaSp-Bar) containing the seed-specific GluC promoter and the Tnos terminator sequence. To generate TR containing the partial AvMaSp gene, pAvMaSp-Bar was introduced into Agrobacterium tumefaciens (EHA105) by electroporation. A modified version of a general rice transformation protocol was used (ToKi, 1997; Mohanty et al., 1999). Rice grains were prepared using a rice milling machine and processed to a fine powder using a blender for oral administration. The compositions of both control (Dong-jin rice) and TR powders were the same, including equal proportions of casein, sucrose, corn oil, cellulose, vitamin mixture, mineral mixture, choline bitartrate, DL-methionine, and t-butylhydroquinone.
Diabetic male BKS.Cg-m+/+Leprdb mice (8 weeks of age) were purchased from Samtako Bio-Korea (Osan, Korea). The mice were housed in a climate-controlled (21±2°C) animal room at a constant 12-h light/dark cycle. All procedures were performed in accordance with protocols approved by the Dong-A University Animal Care and Use Committee (DIACUC-19-13). Each control or TR powder was provided in a separate feeding container ad libitum once per day. After 4 weeks of feeding, the mice (n=15 per group) were sacrificed by carbon dioxide asphyxiation and whole blood was collected by cardiac puncture. The hepatic tissue samples were finely dissected and immediately used for the protein analysis and histological study.
Plasma glucose levels were measured in tail blood using a GlucoDr Blood Glucose Test Strip (Hasuco, Seoul, Korea). Serum insulin concentrations were determined by mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Fujifilm Wako Shibayagi, Gunma, Japan).
Small pieces of abdominal adipose tissues were homogenized in lysis buffer [300 mM NaCl, 0.5% Triton X-100, 50 mM Tris-HCl (pH 7.4), 25 mM NaF, 1 mM Na3VO4, 10 mMNa4P2O7, and protease inhibitor] for 40 min on ice. The lysates were centrifuged at 13,000×g at 4°C for 20 min, the supernatants were collected and protein concentration was measured using the BCA protein assay kit. 30 μg of protein extract with sodium dodecyl sulfate (SDS)-loading buffer was electrophoretically separated on a 8%–15% gradient SDS-PAGE gel, and transferred onto a nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk dissolved in Tris-buffered saline (TBS) buffer containing 0.05% Tween-20 at RT for 1 h. The blots were incubated with primary antibodies, followed by incubation with appropriate HRP-conjugated secondary antibodies. The signals were detected with ECL detection reagent in the LAS-4000 (Fuji, Tokyo, Japan). β-Tubulin was used as internal control for total cellular proteins.
Hepatic tissues were fixed in Bouin’s solution, embedded in paraffin, and sectioned at 5 μm thickness. Tissue sections were placed on glass slides, deparaffinized, hydrated, and treated with 1% hydrogen peroxide (H2O2) for 10min to suppress endogenous peroxidase activity. Antigen retrieval was performed by heating the sections in 1 mM citric acid solution (pH 6.0). Sections were incubated with the primary antibody for STAMP2 at 4°C overnight and reacted with biotinylated horse anti-mouse IgG for 1 h at RT, as per the manufacturer’s instructions for Vectastain Elite ABC kit. The stained sections were developed with liquid diaminobenzidine and counterstained with hematoxylin. The results were observed using a ScanScope digital slide scanning system (Aperio Technologies, Vista, CA, USA).
Data were expressed as the mean±SD of at least 3 independent experiments. The difference in means between 2 groups was analyzed using the Student’s t-test. Mean values were considered significantly different at p<0.05.
We have previously demonstrated that TR-SPF is effective in reducing blood glucose levels in diabetic mice and that concurrent lipolysis in abdominal adipocytes is particularly associated with increased insulin receptor substrate-1 (IRS1) expression and phosphorylation (Park et al., 2019). However, the underlying mechanism by which TR-SPF lowers blood glucose levels in diabetic mice remains to be elucidated at the hepatic level. In this context, we investigated whether feeding diabetic mice TR-SPF causes histopathological changes in their hepatic tissues, as well as alterations in their protein levels, and whether they correlate with insulin resistance in hepatocytes.
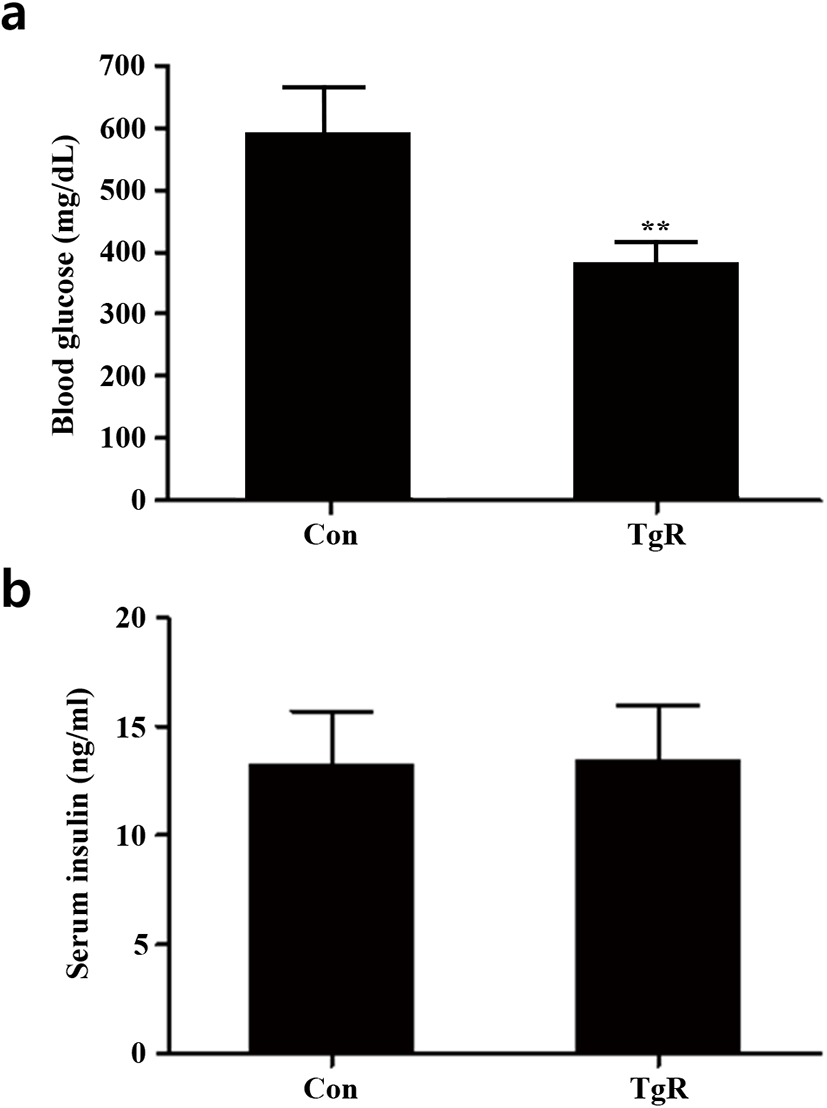
In the diabetic mouse model (BKS.Cg-m+/+Leprdb mice) that was employed, blood glucose levels significantly reduced after exposure to TR-SPF compared with that of the control (Fig. 1a), while serum insulin levels remained unaltered (Fig. 1b). This indicates that dietary exposure to TR-SPF in these diabetic mice only caused a reduction in glucose levels, without significant alterations in serum insulin levels. Thus, it is presumed that TR-SPF might be rather effective to the target tissues for insulin (i.e., the liver and/or adipose tissues) in this animal model.
Recently, silk fibroin (Park et al., 2020), silk protein hydrolyzate (Do et al., 2012), and silk peptides (Park et al., 2020) have been suggested to exert potential antidiabetic effects in diabetic mice (C57BL/KsJ-Leprdb/db) and non-obese type 2 diabetic rats through the regeneration of pancreatic β-cells, an increase in pancreatic β cell mass, and a potentiation of insulin secretion, respectively. Therefore, it is believed that the effect or target of fibroin or silk proteins could differ depending on either diabetic animal models or routes (or methods) of administration to the animals.
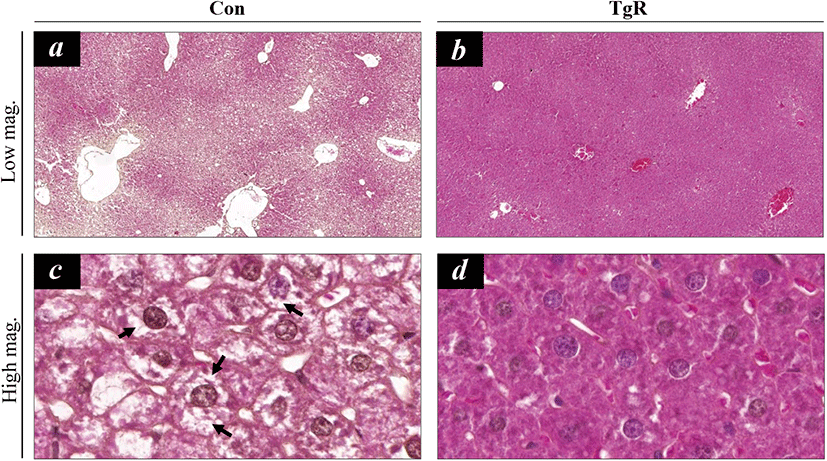
In the present study, hepatic histopathology showed that dietary exposure to TR-SPF significantly alleviated hepatic steatosis (Fig. 2). The hepatocytes in the liver of BKS.Cg-m+/+Leprdb mice mostly retained several lipid droplets in their cytoplasm (Fig. 2a and 2c). However, TR-SPF exposure obviously resulted in disappearance of the cytoplasmic lipid droplets in the hepatocytes (Fig. 2b and 2d), and this indicated that dietary exposure to TR-SPF can provoke a restoration of normal hepatocytes from steatotic hepatocytes. This effect of TR-SPF is comparable to that of metformin, a widely used first-line antidiabetic drug. Metformin is known to ameliorate lipid accumulation in the liver in hepatic steatosis (Woo et al., 2014; Song et al., 2015). The therapeutic effects of metformin are mediated by the activation of AMPK (Hawley et al., 2002; Shaw et al., 2005). In this context, we first monitored the changes in AMPK levels and its phosphorylation in hepatic tissues. However, both AMPK protein content and phosphorylation were consistently downregulated after exposure to TR-SPF (Fig. 3). These results indicate that TR-SPF decreases blood glucose levels and alleviates hepatic steatosis in this animal model through an AMPK-independent pathway.

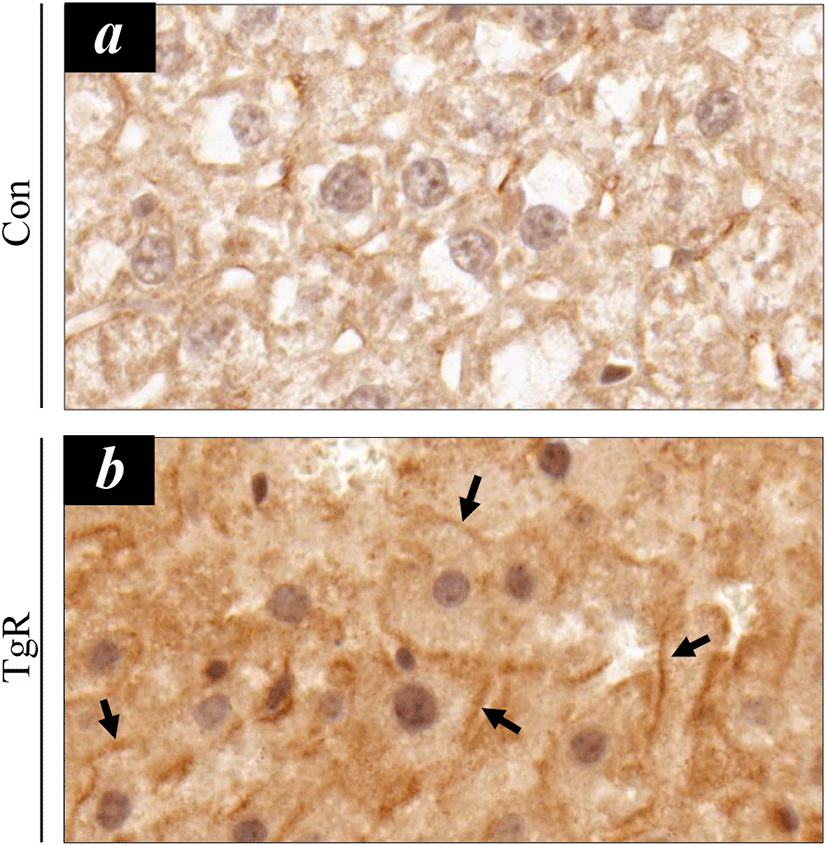
Next, we sought to identify a possible mediator for TR-SPF action and investigated the six-transmembrane protein of prostate 2 (STAMP2) as a potential molecule associated with the alleviation of hepatic steatosis. Previously, it has been shown that STAMP family proteins are associated with adipocyte differentiation (Sikkeland & Saatcioglu, 2013). Furthermore, STAMP2 expression is elevated in fed mice compared to fasting mice (Wellen et al., 2007). Recently, Kim et al. (2015) revealed that hepatic STAMP2 plays an important role in preventing high fat diet (HFD)-induced NAFLD and that STAMP2 overexpression improves hepatic steatosis and insulin resistance in NAFLD. In this study, STAMP2 protein expression was consistently upregulated by TR-SPF exposure (Fig. 3), and STAMP2 upregulation was accompanied by increased Akt phosphorylation (Fig. 3). STAMP2 is known to target the Akt signaling pathway in human adipocytes (Cheng et al., 2011), and it has been reported that administration of antibodies against STAMP2 decreases Akt phosphorylation (Qin et al., 2011). The changes in STAMP2 proteins were confirmed in hepatic tissues using immunohistochemical localization (Fig. 4). Notably, hepatocytes that were immune-positive for STAMP2 were more evident in hepatic tissues from TR-SPF-fed mice (Fig. 4b) compared to the control (Fig. 4a). Furthermore, the STAMP2 proteins were mostly localized in the membrane or boundary of hepatocytes (Fig. 4b). This observation was also consistent with that of a previous study (Korkmaz et al., 2005).
Taken together, this study showed that feeding diabetic mice TR-SPF causes upregulation of STAMP2 expression in hepatic tissues, and thus, alleviates hepatic steatosis. However, it also suggested that AMPK activation may not be required for this cellular process to occur in diabetic mice. We believe that these findings should be confirmed in the other animal models that are used for diabetic research. Finally, the underlying mechanism by which TR-SPF is able to relieve hepatic steatosis in diabetic mice will be understood with greater clarity upon further biochemical and molecular studies in cellular models.

