INTRODUCTION
In general, more specialized or evolutionarily advanced fishes usually have less DNA than the more generalized forms. There are, however, several exceptions that may represent fish groups which are now in the process of evolutionary radiation (Hinegardner & Rosen, 1972). According to Kim et al. (1995), in a given individual, the correlation logically ensues between the nuclear DNA amount and the karyotype, namely the number and shape of the chromosome characteristic of the species. In general, studies of nuclear DNA content in living organisms in fish particularly have been performed using Feulgen-stained blood smears and microdensitometry analysis (Oliveira et al., 1992, 1993a, 1993b; Carvalho et al., 2002), or blood cell samples in suspension, which were stained with base-specific fluorochromes and analyzed by flow cytometry (Tiersch et al., 1989a, 1989b, 1990; Carvalho et al., 2002).
Flow cytometry has various forms of clinical applications in oncology for understanding surface expression, intracellular signaling, cell cycle content analysis, and a number of other interesting parameters (Vanparys et al., 2006; Park, 2019, 2020; Park & Choi 2020). Recent developments in instrument platforms, calibration methods, and reagent quality have now made flow cytometry a valuable tool for DNA content analysis (Estevam et al., 2011; Goo et al., 2018; Park, 2019, 2020; Park & Choi 2020). These calibration packages can detect whether the parameters are within acceptable ranges and thus allow for consistent sample acquisition over time. One of the advantages of flow cytometry is the promptness of the measurement, making it possible to measure thousands of cells over a short period of time, and the ability for multi-color immunophenotyping (Estevam et al., 2011; Goo et al., 2018).
However, no fishes have been examined to have their nuclear DNA content so far among 200 species of endemic fish species that live in the rivers of Korea (Byeon et al., 2010). The enormous diversity of nuclear DNA content observed among fish led Ohno (1974) to suggest that comparative studies of the karyotype of different fish groups would make no sense if they were not followed by the information regarding change in genome size. This work is based on data gathered from analyses of 31 endemic species of fishes that appear in the river of Korea and is part of an overall study of cellular DNA content in the animal kingdom.
MATERIALS AND METHODS
On June 2019, samples of 31 endemic species (16 Cyprinidae, 13 Cobitidae, 1 Cottidae, and 1 Amblycipitidae) were trapped in the Hantan River (Fig. 1a: Gomun-ri,Yeoncheon-eup, Yeoncheon-gun, Gyeonggi-do, Korea [38° 03′ 41.29″N, 127° 07′ 20.80″E]), Imjin River (Fig. 1b: Wondang-ri, Jangnam-myeon, Yeoncheon-gun, Gyeonggi-do, Korea [37° 57′ 57.82″N, 126° 53′ 15.11″E]), Han River (Fig. 1c: Haengjue-dong, Deokyang-gu, Goyang-city, Gyeonggi-do, Korea [37° 35′ 31.13″N, 126° 49′ 09.56″E]), Kum River (Fig. 1d: Seochang-ri, Gyeonggi-do, Korea [37° 35′ 31.13″N, 126° 49′ 09.56″E]), Yongsan River (Fig. 1e: Sinhak-ri, Sihong-myeon, Yeongam-gun, Jeollanam-do, Korea [34° 48′ 35.44″N, 126° 36′ 17.67″E]), and Nakdong River (Fig. 1f: Doyo-ri, Saengnim-myeon, Gimhae-city, Gyeongsangnam-do, Korea [35° 21′ 55.21″N, 128° 53′ 13.39″E]) (Fig. 1, According to Goo et al., 2018). The measurement of standard length and body weight were taken to the nearest 0.01 cm and 0.01 g using digital vernier calipers (CD-20CP; Mitutoyo, Kawasaki, Japan) and electronic balance (Shimadzu, Kyoto, Japan) in all samples. Collected samples fixed in 70% ethanol (Sigma-Aldrich, St. Louis, MO,USA) and 70% ethanol of each sample were exchanged after 24 hours.
DNA content analysis was performed using flow cytometry (PA-Ⅱ, Partec, Munich, Germany). Ventral fins from each fishes were analyzed using flow cytometry measurement. For flow cytometric analysis, tissues of ventral fin were homogenized and filted using 30 µm filter, after that centrifugation (Centrifuge Micro 17R, Hanil Science Industrial, Incheon, Korea; 12,000×g, 10 min). And removed supernatant liquid and added 0.5 mL CyStain DNA 2 step nuclei extraction buffer (CyStain DNA 2 step high resolution DNA staining kit, Partec) and 2 mL CyStain DNA 2 step staining buffer (CyStain DNA 2 step high resolution DNA staining kit, Partec).
The tail fin tissue with 1.7 pg DNA/nucleus of marine medaka, Oryzias dancena were used as a standard reference (Park et al., 2016; Park, 2021) in this experiment. The marine medaka isn’t indigenous to Korea. However, this species is accredited by the Ministry of Land, Transport and Maritime Affairs, Korea (Ordinance of Agriculture, Food and Fisheries, No. 1) and is imported legally from Indonesia (Kim et al., 2009a, 2009b; Park, 2021). The experiment was performed in triplicate and the results are reported as means±SE (n=50), unless otherwise stated.
RESULTS AND DISCUSSION
Hinegardner & Rosen (1972) proposed the following combination of items to explain the noted distribution of DNA in the Teleostei. There are three possible changes that could be associated with DNA during the evolution; (1) The amount of DNA could remain constant and be modified by mutation or recombination, (2) it could increase by duplication, or (3) it could decrease. All these changes have occurred, both separately and in combination. A combination of mutation, recombination, and natural selection is perhaps the foundation for speciation (Hinegardner & Rosen, 1972).
The results obtained among the species of 31 analyzed endemic species are shown in Table 1 and Fig. 2. DNA contents of 31 endemic species were observed to span from 1.5 to 4.8 pg DNA/nucleus. As shown in Table 1 and Fig. 2, In Cyprinidae, DNA content of Abbottina springeri (1.5±0.03 pg DNA/nucleus) was the lowest value in all experimental groups and DNA content of Acheilognathus somjinensis (1.9±0.04 pg DNA/nucleus) was higher than that of A. springeri. DNA content of Carassius cuvieri (4.5±0.32 pg DNA/nucleus) was the highest value in Cyprinidae groups. DNA content of Carassius cuvieri were higher than those of C. auratus (3.8±0.41 pg DNA/nucleus) and Cyprinus carpio (3.6±0.24 pg DNA/nucleus). DNA content of C. auratus was higher than that of C. carpio, and DNA content of Abbottina rivularis (3.9±0.18 pg DNA/nucleus) was higher than those of C. auratus and C. carpio.
Each value are average values of triplicate experiments (n=50).
DNA contents (pg/nucleus): Standard control used was fin cell of marine medaka, Oryzias dancena (1.71 pg/nucleus: According to Park et al., 2016).
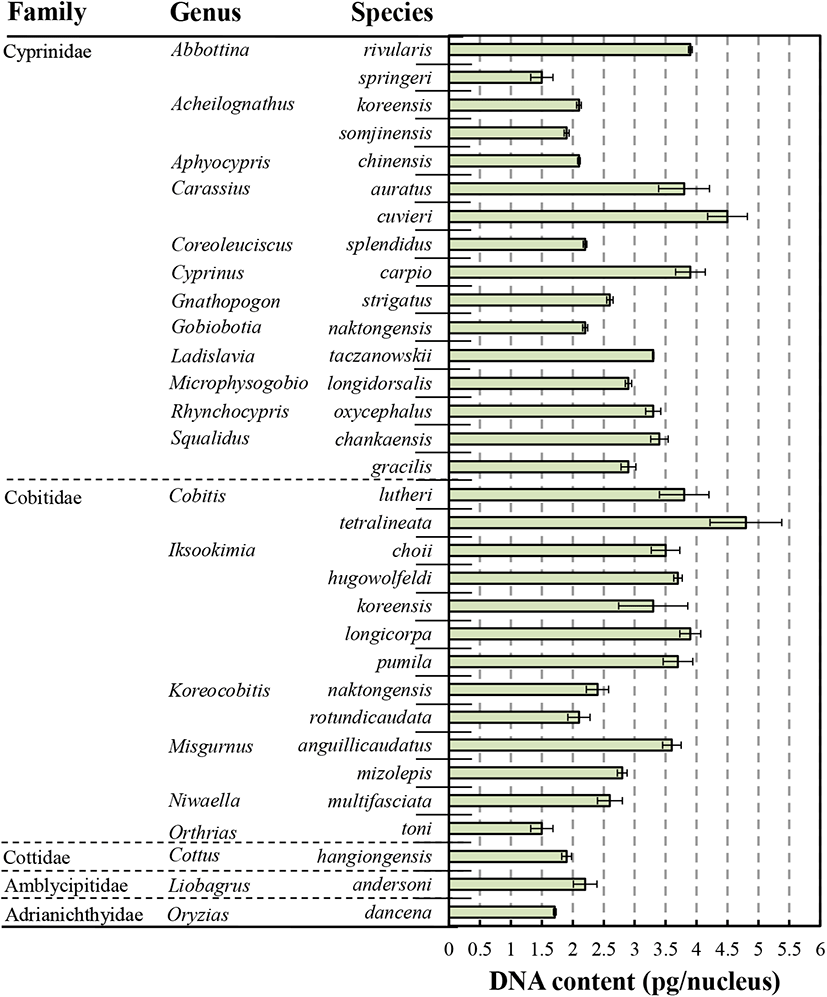
As mentioned by Kim et al. (1995), Misgurnus mizolepis has 2n=48 chromosomes, being made of 12 metacentric (M), 4 submetacentric (SM), and 32 acrocentric (A) chromosomes, and M. anguillicaudatus has 2n=50 chromosome, consisting of 10 M, 4 SM, and 36 A chromosomes. Both species has an arm number (NF) of 64. As mentioned Nam et al. (1998), Cyprinus carpio had 2n=100 diploid chromosomes, comprising 12 M, 40 SM, and 48 A chromosomes, and diploid chromosome number of Carassius auratus was also 2n=100, just as the karyotype of C. carpio and C. auratus, which has 12 M, 36 SM, and 52 A chromosomes in Japan. DNA contents of M. anguillicaudatus and C. carpio were higher than those of M. mizolepis and C. auratus, respectively. Metacentric chromosome numbers of M. anguillicaudatus and C. carpio were lower than those of M. mizolepis and C. auratus respectively. In addition, acrocentrics numbers of M. anguillicaudatus and C. carpio were higher than those of M. mizolepis and C. auratus.
As shown in Table 1 and Fig. 2, in Cobitidae, DNA content of Cobitis tetralineata was the highest value (4.8±0.58 pg DNA/nucleus) and Orthrias toni was the lowest value (1.5±0.18 pg DNA/nucleus) in all experimental groups. In genus Iksookimia, DNA content of 5 species were observed to rang from 3.3 pg DNA/nucleus (I. koreensis) to 3.9 pg DNA/nucleus (I. longicorpa). In genus Misgurnus, DNA content of M. anguillicaudatus (3.6±0.15 pg DNA/nucleus) was lower than that of M. mizolepis (2.8±0.08 pg DNA/nucleus).
DNA contents of Cottus hangiongensis in Cobitidae and Liobagrus andersoni in Amblycipitidae were 1.9±0.08 pg DNA/nucleus and 2.2±0.19 pg DNA/nucleus each (Table 1 and Fig. 2). As a result, DNA content was observed to range from 1.5 to 4.8 pg DNA/nucleus in all freshwater fishes in Korea. In the same family and the same genus groups, DNA contents of larger species don’t have higher value than those of smaller species in every case. So, DNA content was unrelated to morphometric characteristics (standard length and body weight) in the same family and the same genus groups.
Among vertebrates, DNA content has been observed to span from 0.78 to 280.00 pg per diploid nucleus (Olmo et al., 1989; Carvalho et al., 2002). Among fishes, DNA content spans from 0.78 pg per diploid nucleus in Tetraodon fluviatis (Hinegardner & Rosen, 1972; Carvalho et al., 2002) to 248.00 pg in Lepidosiren paradoxa (Ohno & Atkin, 1966; Carvalho et al., 2002). The data acquired by Hinegardner & Rosen (1972) on 275 teleostei showed that there is a clear modal value of about 2.0 pg of DNA per diploid nucleus. However, the differences greater over twice in DNA content may be found among specimens of the same family or even of the same genus, as is the case of Cyprinids of the genus Barbus (Ohno et al., 1967; Wolf et al., 1969; Carvalho et al., 2002) and species of the genus Corydoras (Hinegardner & Rosen, 1972; Oliveira et al., 1992; Carvalho et al., 2002). Although some authors have suggested that this variation should be related to the number of genes in the organisms or to the complexity of their development (Cavalier-Smith, 1978; Carvalho et al., 2002), many agree that there is no significant correlation between the amount of nuclear DNA and the organic or genetic complexity (Cavalier-Smith, 1978; Carvalho et al., 2002). In addition, this study is of no significant correlation between DNA content and organic complexity (standard length and body weight).
As mentioned by Hardie & Hebert (2004), genome size diversity in ray finned fishes is not related to metabolic rate and organic complexity, but is obviously correlated with egg diameter, suggesting linkages to the evolution of parental care. Hinegardner & Rosen (1972) have observed that specialized fishes had smaller genomes than more generalized forms, and this trend was supported in the subsequent studies (Cimino, 1974). This pattern may reflect decreases in chromosome number and genome size in specialized species, or constraints on genome size related to the heightened developmental complexity of specialized species (Hardie & Hebert, 2004). Thus, the analysis of nuclear DNA content raises a problem for evolutionary genetics regarding the interpretation of this quantitative variation in genome size or in DNA amount (Gold & Price, 1985; Carvalho et al., 2002).
Our current investigation can be utilized as a foundation for continued cytogenetic studies of freshwater fishes in Korea for species classification or maintenance, as well as to provide basic data for commercial production of new species. Moreover, further extensive studies are needed to get more complete information on the evolutionary direction and detailed genetic relationship among freshwater fishes in Korea by conducting chromosome analysis, detecting nucleolus-organizer regions, and analyzing chromosome in situ hybridization.
CONCLUSION
In conclusion, this result suggests that DNA content is related to chromosome number. However, I am not entirely sure of this conclusion, because the other 27 endemic species from 31 species in this study inhabiting were not fully analyzed karyotype yet. So, future research need to investigate unknown karyotype analysis of 27 endemic species in Korea, and the investigation of relationship between chromosome and DNA content is necessary as well.

