INTRODUCTION
One of assisted reproductive technologies (ART) in domestic animal industry, liquid- or cryo- preservation of sperm are used for artificial insemination (AI), maintenance and supply of the superior genetic resources, and decrease the possibility of diseases. In the pig industry, liquid preserved semen are commonly used, however, they are hard to keep for long time because they are exposed to various stresses such as light, temperature and chemicals that caused reduction of sperm viability and fertility (Kadirvel et al., 2009; Alkmin et al., 2014). To enhance the preservation periods of boar sperm, cryopreservation was developed and cryopreserved sperm have prolonged preservation periods compared to liquid preserved sperm (Yoshida, 2000). Although preservation period of sperm is prolonged, spermatozoa was damaged by cold shock, oxidative stress, and ice crystal formation during freezing and thawing process, and these damages negatively influence to membrane function and structure of spermatozoa via change of ratio between phospholipid and cholesterol in plasma membrane (White, 1993; Agarwal et al., 2014; Baishya et al., 2014). Therefore, numerous researches have been carried out to reduce cryodamage by supplementation of antioxidants (Kaeoket et al., 2010), amino acids (Reddy et al., 2010) and cholesterol (Moce et al., 2010) during cryopreservation.
Because sperm membrane contains various polyunsaturated fatty acids (PUFAs), membrane integrity of spermatozoa and supplementation of fatty acid are closely associated. Safarinejad et al. (2010) had reported that concentrations of omega-3 (n-3) fatty acids including alpha-linolenic acids (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) in spermatozoa from fertile men were higher than sperm from infertile men, while omega-6 fatty acids were lower in sperm from fertile men. These n-3 fatty acids were found in fish oil and sunflower oil that are used as one of feed composition and dietary supplement of these oil influenced to sperm characteristics. In bulls, intake of sunflower oil enhanced motility and plasma membrane integrity of frozen-thawed spermatozoa (Adeel et al., 2009). And progressive motility was increased in cooled and frozen sperm from DHA-enriched nutriceutical fed stallion compared to control diet (Brinsko et al., 2005). Rooke et al. (2001) reported that supplementation of tuna oil to boar diets increased proportion of progressively motile sperm and normal acrosome, whereas abnormal morphologies were decreased. These results demonstrated that supplement of fatty acids could improve membrane integrity, sperm motility and viability, as well as cold sensitivity.
As one of phospholipid in plasma membrane, ALA plays role as precursor of EPA and DHA, energy resource, and regulator of membrane function (Shevchenko & Simons, 2010). In our previous study, we found that addition of 3 ng/mL of ALA in frozen extender protected ethanol-mediated damages on plasma membrane and acrosome during cryopreservation of boar spermatozoa (Lee et al., 2016b). Freezing and thawing process lead to discharge phospholipid such as ALA and arachidonic acid in plasma membrane of spermatozoa and it was caused to lose the membrane function and stability. Therefore, we expected that supplementation of ALA during freezing process could reduce the membrane damages by discharging of phospholipids in plasma membrane. However, Graham & Foote (1987) demonstrated that hydrophilic outer side of phospholipid bilayer is cause to low solubility and efficiency of lipids supplementation. For this reason, ALA was recommended to use with carrier proteins such as bovine serum albumin (BSA) and methyl-beta-cyclodextrin (MBCD). Based on these reports and our previous studies (Lee et al., 2016a; 2016b), we hypothesized that supplementation of ALA combined with carrier proteins could protect membrane damages through increasing of its solubility and efficiency during cryopreservation of boar sperm. Therefore, this study was conducted to investigate protective effect of ALA combined with BSA or MBCD on plasma and acrosomal membrane, morphology and oxidative stress in boar spermatozoa.
MATERIALS AND METHODS
All procedures that involved the use of animals were approved by the Kangwon National University Institutional Animal Care and Use Committee (KIACUC-09-0139). All of boar semen were purchased from Gumbo (Wonju, Korea). The semen were collected by gloved-hand method once a week and transported to laboratory. The sperm samples with more than 75% motility and 80% viability were used for this study.
All of reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). For supplementation of ALA into the first freezing extender, ALA was mixed with same molar ratio of BSA or MBCD. Briefly, 0.06 mg of ALA was added to 10 mL BSA or MBCD solution (1.4 mg/mL and 0.028 mg/mL, respectively) diluted at distilled water and each mixtures were stirred at room temperature for 1 h. Then, 0.5 µL of aliquot was supplied to 1 mL of first freezing extender. Based on our previous results (Lee et al., 2016b), 3 ng/mL of ALA was determined as final concentration. To clarify effect of ALA mixture, 3 ng/mL ALA and same molar ratio of BSA or MBCD was added to first freezing extender, respectively.
As a first freezing extender, lactose egg yolk (LEY; 11% [w/v] lactose and 20% [v/v] egg yolk in distilled water) was used. The boar sperm was diluted at 1.5×109 spermatozoa/mL using 1mL of LEY containing ALA combined with BSA or MBCD and it was cooled down to 4℃ for 2 h. Then, 0.5 mL of LEY supplemented with 9% Glycerol and 1.5% Orvus Es Paste (OEP; Nova Chem, USA) was added to cooled sperm samples and they were injected into 0.25 mL straws. Filled straws were placed on 10 cmabove liquid nitrogen (LN2) for 10 min, then, pre-freezing samples were immersed and preserved in LN2. To analyze sperm characteristics, cryopreserved samples were thawed at 37℃ for 45 sec.
Flow cytometry was used to measure plasma membrane intact, acrosomal damage, mitochondrial activity, reactive oxygen species (ROS) level and lipid peroxidation in frozen-thawed boar sperm. The data from flow cytometry were obtained from total 10,000 of spermatozoa using FACsCalibur and were analyzed by CELLQuest version 6.0.
To measure intact of plasma membrane, 6 nM SYBR-14 was used, and 3 μM Lectin from Arachis hypogagea (FITC-PNA) and 2 μM Rhodamine123 were used to analyze acrosomal damage and mitochondrial activity, respectively. Frozen-thawed sperm samples were stained using each fluorescent dye at 38℃ in dark room for 5 min. Then, 2 μM propidium iodide was added to stained samples and subsequently incubated in same condition for 5min.
Intracellular ROS level in live spermatozoa was measured using H2DCFDA (0.8 µM, Molecular probes, USA). Thawed sperm was stained by H2DCFDA at 38℃in dark room for 15 min. Then, 2 μM propidium iodide was added to stained samples and subsequently incubated in same condition for 5min.
The frozen-thawed semen was diluted at 4×106 spermatozoa/mL with modified Modena B (30 g/L glucose, 2.25 g/L EDTA, 2.5 g/L sodium citrate, 1 g/L sodium bicarbonate, 5.00g/L tris, 2.5 g/L citric acid, 0.05 g/L cysteine and 0.3 g/L gentamicin sulfate). Then, 7 µL of semen sample was placed onto a pre-warmed glass slide (37.5℃) and it was covered by cover slip. To maintain sperm motility, samples were placed on 37.5℃ warm stage and a total of 200 spermatozoa in randomly selected five section were observed using light microscope. The sperm with tail movement was decided as motile sperm.
Rose Bengalstaining was used to observe morphological abnormality of frozen-thawed boar spermatozoa. The samples were smeared on slide glass and were dehydrated at room temperature. Then, samples on slide glass were stained using 3% (v/v) Rose Bengal solution and excessive Rose Bengal solution was removed. After dehydration of stained sperm, retained Rose Bengal was washed using water and sperm samples were dehydrated for observation. Morphological abnormality of sperm was classified by following criteria; in head:detach, big head, small head, and twin head; in middle piece:cytoplasmic droplet, folded, and absence; in tail:folded, coiled, short tail, and absence. The sperm with abnormal morphology were observed under a light microscopic at ×400 magnification and a total of 200 spermatozoa in randomly selected five section were counted.
RESULTS
Changes of membrane integrity, mitochondrial activity and morphologically abnormal sperm were presented in Table 1. Both of carrier proteins combined with ALA enhanced plasma membrane intact and mitochondrial activity, and reduced acrosomal membrane damage and sperm abnormalities compared to control group (p<0.05). In particular, treatment of ALA combined with MBCD improved plasma and acrosomal membrane potential compared with single treatment of ALA, BSA and MBCD (p<0.05). Interestingly, acrosomal damage was decreased by ALA and carrier proteins regardless of combination (p<0.05).
Control, without ALA, BSA, and MBCD; ALA, with 3 ng/mL ALA; BSA, with 0.7 µg/mL BSA; MBCD, with 14 ng/mL MBCD; ALA+BSA, with 3 ng/mL ALA and 0.7 µg/mL BSA; ALA+MBCD, with 3 ng/mL ALA and 14 ng/mL MBCD.
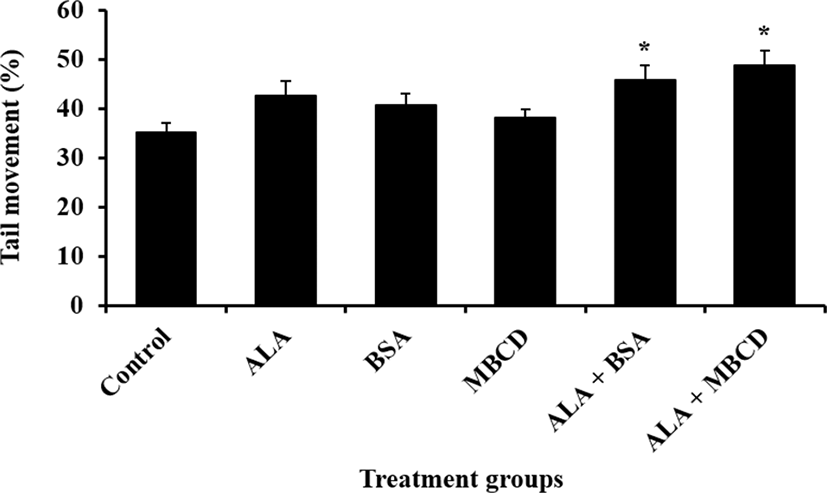
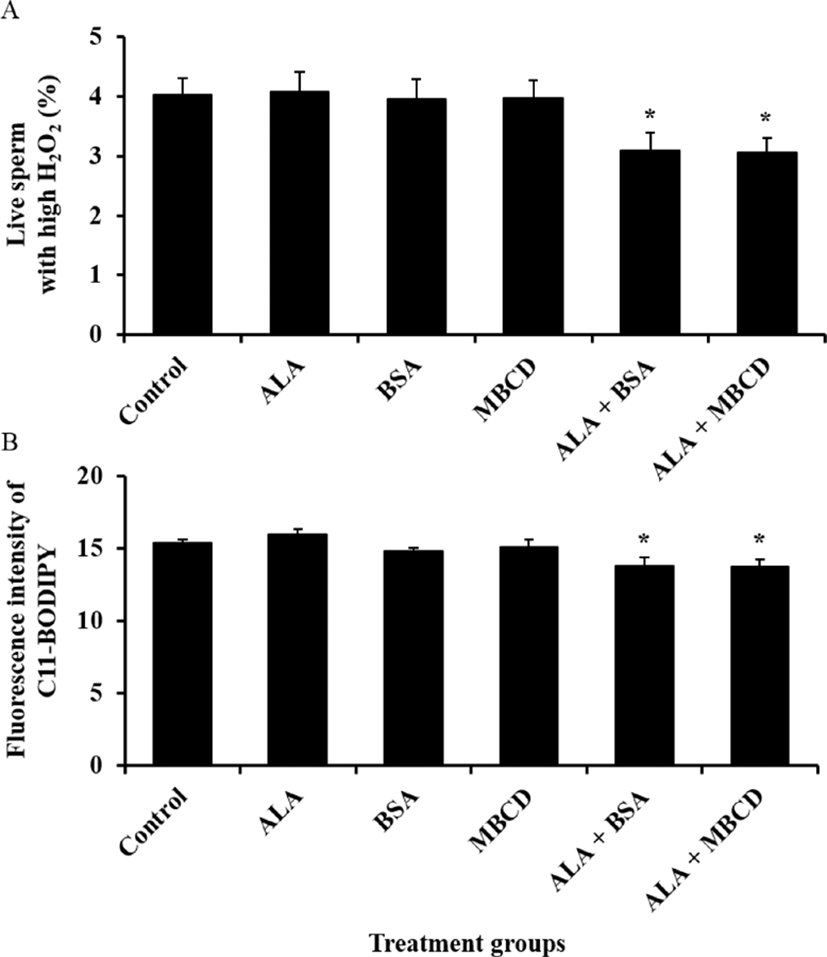
Effect of ALA combined with BSA or MBCD on motility and oxidative stress status in frozen-thawed boar sperm were showed in Fig. 1 and 2, respectively. The ratio of sperm with tail movement in ALA combined with both of carrier proteins were higher than control (p<0.05). Both of live sperm with high level of ROS and LPO were reduced by supplementation of ALA combined with both of BSA and MBCD compared to control group (p<0.05).
DISCUSSION
This study was conducted to confirm effect of ALA combined with carrier proteins during cryopreservation on membrane damage, morphological abnormality, motility and oxidative stress status of boar sperm. The findings in this study show that 1) ALA combined with BSA and MBCD during cryopreservation reduced plasma and acrosomal membrane damages, and 2) reduced membrane damage improved mitochondrial activity, morphological intact and motility. Finally, 3) treatment of ALA combined with carrier proteins could decreased LPO derived from ROS generation.
As one of detrimental factor during freezing of spermatozoa, change of membrane structure is occurred by redistribution of lipids and proteins in sperm plasma membrane during cooling process at from 22℃ to 1℃ (Parks & Graham, 1992). During the temperature change, fatty acids that caused to reduce membrane fluidity and permeability are released from plasma membrane (Watson, 2000). Kaka et al. (2015) reported that supplementation of ALA with ethyl alcohol during cryopreservation of bull sperm increased ALA concentration in spermatozoa and improved motility, membrane and acrosome integrity, and viability. However, because outer side of plasma membrane have hydrophilic property, fatty acids including ALA is hard to pass through membrane. Therefore, carrier proteins such as BSA and MBCD are commonly used to enhance efficiency of fat-soluble substrates in various researches. The BSA combined with oleic and linoleic acids promoted growth activity of human diploid fibroblasts (Kan & Yamane, 1982) and supplementation of cyclodextrin combined fat-soluble substrates including cholesterol and vitamin E improved quality of frozen-thawed ram sperm (Benhenia et al., 2016; Benhenia et al., 2018).
Both of plasma membrane and mitochondria of spermatozoa play a crucial roles in motility, fluidity and viability, however, they were damaged by a variety of damages that are occurred during freezing and thawing process (Watson, 2000). In particular, mitochondria is involved in production of energy and movement of sperm flagellum (Cardullo & Baltz, 1991). Their functions are closely related to sperm motility and motility of sperm is important parameter for quality because sperm with high motility has a high probability of fertilization with oocyte (Talevi et al., 2013). Therefore, reduction of damage on plasma membrane and mitochondria is most important to maintain the sperm viability, motility and function, and a variety of substrates such as fatty acids, saccharides and antioxidants were added to freezing extender for reduction of cryo-stress (Pena et al., 2003; Bucak et al., 2007; Kaka et al., 2015). In present study, supplementation of ALA combined with BSA and MBCD enhanced the plasma membrane intact, mitochondrial activity and sperm motility, whereas single treatment of ALA, BSA and MBCD did not affect compared to control group. These results were corresponded to our previous results that treatment of diluted ALA using 0.1% BSA increased viability and mitochondrial intact of frozen-thawed boar spermatozoa compared to non-treated group, however, individual treatment did not differ (Lee et al., 2016a). The addition of n-3 ALA to extender elevated PUFA content of sperm membrane and this led to improve the fluidity of sperm membrane and increase sperm motility (Towhidi & Parks, 2012). Thus, we expected that treatment of ALA with carrier proteins could enhanced effect of fatty acid on function and motility of spermatozoa via increasing absorption into plasma membrane surrounding the spermatozoa including head, middle piece and tail. However, ALA concentration in sperm by using of carrier proteins is needed to measure.
The ice crystal formation during cryopreservation of sperm is one of detrimental factors of changes of membrane composition including re-aggregation of phospholipids and redistribution of the membrane domain (Muller et al., 2008). These changes in sperm membrane led to alteration of membrane permeability, intracellular calcium concentration and morphology (Parks & Lynch, 1992). Especially, intact plasma membrane in spermatozoa is important for maintenance of their morphology and efflux of phospholipids in plasma membrane during cryopreservation is caused the morphological abnormalities (Brinsko et al., 2005). In our present findings, morphologically abnormality sperm in ALA with both of carrier proteins groups were lower than control group and it was corresponded with result of plasma membrane intact. In bull, treatment of ALA during freezing process increased ratio of normal morphology of spermatozoa after thawing and these tendency of morphological results was corresponded with results in membrane integrity and motility (Kaka et al., 2015). Therefore, these results suggest that supplementation of ALA with BSA or MBCD could replace released phospholipids from plasma membrane, and it contributed stabilization of membranes integrity and structure against cold shock.
Acrosome reaction is an essential phenomenon for penetration of sperm into the oocyte and acrosome reacted sperm before access to oocytes lost their fertility and viability (Bleil et al., 1988).Because the first step of acrosome reaction is fusion of plasma and acrosomal membrane, maintenance of membrane integrity in spermatozoa is closely associated with prevention of early acrosome reaction. It is induced by various physical and chemical stress such as ROS generation, ice crystal formation and osmotic pressure (Lamirande et al., 1998). In this present study, acrosomal damage was decreased by ALA, BSA and MBCD regardless of complex treatment, in particular, ALA combined with MBCD showed lowest acrosomal damage. These results was similar to our previous study that treatment of ALA, BSA and ALA diluted by BSA reduced acrosome reacted sperm (Lee et al., 2016a). These our results showed that acrosomal membrane was more sensitively affected to ALA and carrier proteins than plasma membrane and mitochondrial activity.
Because plasma membrane of boar spermatozoa contains higher concentration of PUFAs than other species, they are more sensitive to damage from ROS and ROS-induced LPO (Tavilani et al., 2006). The efflux of PUFAs from plasma membrane is caused to ROS generation and LPO, and induced LPO repeatedly effuse fatty acids in plasma membrane though activation of phospholipase A2 (Wathes et al., 2007). These efflux of fatty acids, generation ROS and LPO lead to damage on mitochondrial membrane and DNA, loss of motility, acrosome integrity and viability (Schiller et al., 2000; O'Connell et al., 2002; Kadirvel et al., 2009). Kaka et al. (2015) reported that malondialdehyde levels in frozen-thawed bull sperm that is used for measurement of LPO were dose-dependently increased by ALA diluted with ethanol and this results indicated that high concentration of ALA during cryopreservation of sperm could induce LPO. In our findings, intracellular H2O2 level in live sperm and LPO were decreased by ALA combined with both of BSA and MBCD compared to non-treated group. We used final concentration of 3 ng/mL ALA in this study, and expected that this ALA concentration did not induced LPO in frozen-thawed boar sperm. Therefore, these results suggest that low concentration of ALA with carrier proteins could reduce ROS generation and ROS-induced LPO without increasing of LPO through stabilization of plasma membrane.
In conclusion, we found that treatment of ALA combined with carrier proteins during cryopreservation enhanced plasma and acrosomal membrane intact, mitochondrial activity, motility, and morphology, and reduced intracellular ROS and LPO levels in frozen-thawed boar spermatozoa. These findings suggest that carrier proteins such as BSA and MBCD could enhanced efficiency of ALA during freezing process of sperm, and ALA combined carrier proteins could protect membrane and functional integrity of sperm, and morphology through reduction of ROS generation and LPO by stabilization of plasma membrane.

