INTRODUCTION
Because pigs have anatomical and physiological similarities with human, they are considered as useful animal model for xenotransplantation, stem cell research model, and human disease model. Therefore, establishment and improvement of optimal in vitro production (IVP) system are very important for various researches (Sovernigo et al., 2017), however, quality of in vitro-produced embryos is still lower than in vivo (Gilchrist, 2010). To improve low efficiency of IVP system, a variety of substances, such as vitamin, antioxidants, and hormoens, have been supplied to medium for IVP of oocytes (Kwak et al., 2012; Tareq et al.,2012; Li et al., 2017).
Polyspermy is a major obstacle for IVP of porcine embryos and frequency of polyspermic fertilization was higher in in vitro than in vivo (Reis, 1993; Hoodbhoy and Talbot, 1994; Li et al., 2003). During the maturation process of oocytes in vitro, nuclear maturation of porcine oocytes was faster than cytoplasmic maturation and these mismatch between cytoplasmic and nuclear maturation was caused incomplete maturation status of oocytes and inducing of polyspermy, resulting a lower rate of in vitro development after fertilization (Wang et al., 1997; Elahi et al., 2016). Therefore, regulation of both cytoplasm and nuclear maturation is very important to prevent polyspermy.
Recently, many studies have been conducted to determine whether dietary intake of polyunsaturated fatty acid (PUFA) in cattle could improve reproductive performance. Childs et al. (2008) reproted that PUFAs supplement by diet in cattle increased proportion of PUFAs in follicular fluids and Fouladi-Nashta et al. (2007) reproted that developemental competence of oocytes and quality of blastocyst were enhanced by dietary PUFAs in cows. These reseaches suggested that supplementation of fatty acids (FAs) could improve quality and developmental potential of mammalian oocytes.
Alpha-linolenic acid (ALA), is one of PUFAs, is present in follicles, oocytes, and spermatozoa in mammals (Homa and Brown, 1992). Marei et al. (2009) reported that addition of ALA in maturation medium for bovine oocytes enhanced clevage rate, blastocyst formation rate, and embryo quality. Our previous study also showed that the treatment of 50 μM ALA during in vitro maturation (IVM) of porcine oocytes significantly increased the metaphase-II reached oocytes, and cleavage rate and numbe of cells in blastocysts were increased by ALA treatment during in vitro culture (IVC) (Lee et al., 2017a). Therefore, we hypothesized that addtion of ALA during maturation of mammalian oocytes have benefical effect as an energy sourse and this study was conducted to evaluate effect of ALA during IVM on improvement of fertilization and developmental competence of porcine oocytes via cumulus expansion. Futhermore, the expression of FA metabolism- and cumulus expansion-related genes were measured.
MATERIALS AND METHODS
All procedures that involved the use of animals were approved by the Kangwon National University Institutional Animal Care and Use Committee (KIACUC-09-0139). Ovaries were collected from local slaughterhouse and transferred to the laboratory in 0.9% (w/v) sterilized saline within 2 h. The cumulus-oocyte complexes (COCs) were aspirated from antral follicles (3–6 mm diameter) using 18-gauge needle with 10 cc syringe. After aspiration, COCs with compact cumulus layer and homogeneous cytoplasm were selected and incubated in medium-199 (Invitrogen, MA, USA) containing 10% (v/v) porcine follicular fluid (pFF), 10 IU/ mL human chorionic gonadotropin (hCG; Intervet), 10 µg/mL luteinizing hormone (LH; Sigma-aldrich, St. Louis, MO, USA), 0.5 µg/mL follicle stimulating hormone (FSH; Sigma-Aldrich) and 10 ng/mL epidermal growth factor (EGF; Sigma-aldrich) with 0, 25, 50, and 100 μM ALA at 38.5° in 5% CO2 for 20–22 h. Then, they were subsequently incubated in hormone-free maturation medium for 20–22 h.
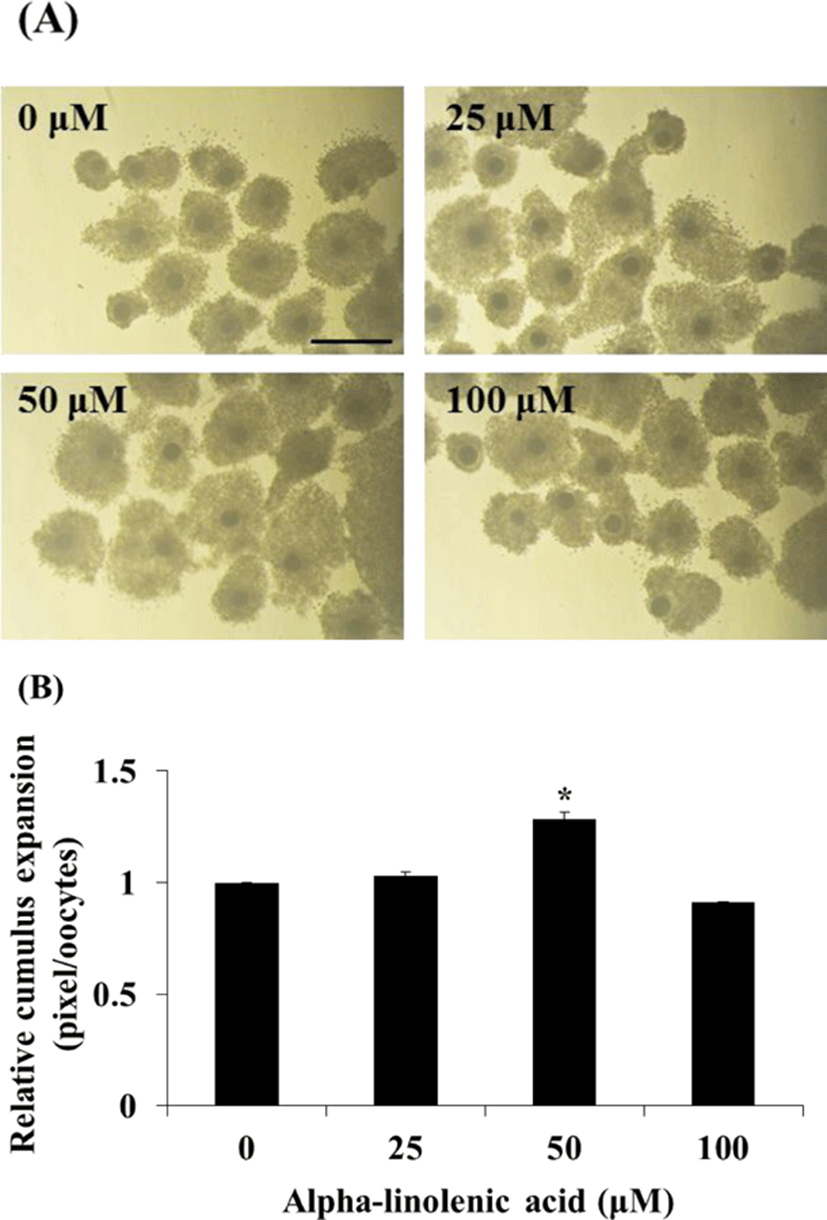
To assess effect of ALA treatment on cumulus expansion, COCs were incubated with different concentration of ALA and area of COCs was observed using inverted microscope at 22 h after maturation. Then, cumulus area from 50 COCs in each treatment groups was measured using Image J software (Version 1.46; National Institutes of Health, Bethesda, MD, USA) and was normalized to control group.
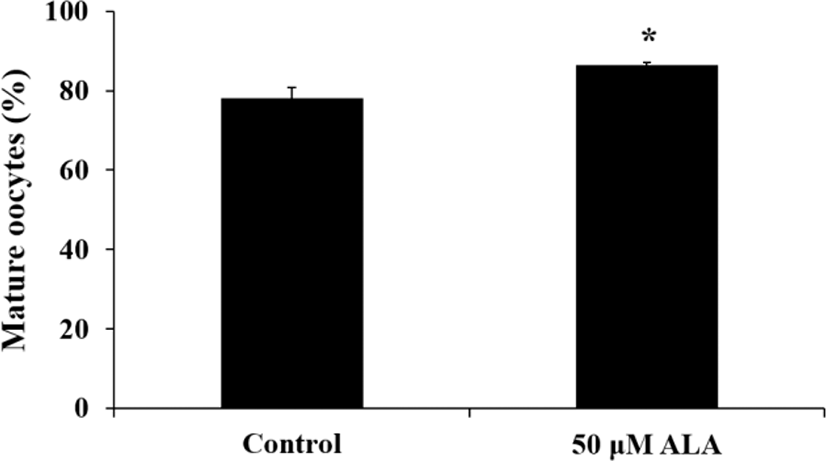
Aceto-orcein stain method was used to evaluate nuclear maturation stage of porcine oocytes. The oocytes were incubated with 50 μM ALA during IVM. The cumulus cells were removed by gentle pipetting with 0.1% hyaluronidase and denuded oocytes were fixed in fixation solution (acetic acid : ethanol; 1 : 3; v/v) at room temperature (RT) for 48 h and nuclear in oocytes was stained by 1% (w/v) aceto-orecein for 7 min. The morphology of nuclear was observed under light microscope. The oocytes at germinal vesicle (GV), germinal vesicle breakdown (GVBD), metaphase I (MI), anaphase (AI) and telophase I (TI) phase were decided as immature oocyte and metaphase II (MII) stage were classified as mature oocytes.
The cumulus cells surrounding COCs mature with different concentration of ALA were removed by gentle pipetting with 0.1% hyaluronidase. And 15–20 oocytes were placed in 50 μl drop of modified tris-buffered medium (mTBM) containing 0.2% (w/v) bovine serum albumin (BSA; Sigma-Aldrich). Boar semen were washed twice using modena B and were diluted with mTBM containing 0.4% (w/v) BSA and 0.39 mg/mL caffeine (Sigma-Aldrich) at a final concentration of 6×105 spermatozoa/mL. Then, 50 µL aliquots of sperm were added and co-incubated with denuded oocytes for 18–20 h.
To evaluate sperm penetration into oocytes, aceto-orcein stain was used. The spermatozoa surrounding putative zygotes were removed and zygotes were fixed in fixation solution for 48 h. Then, they were stained by 1% aceto-orcein solution for 7 min and observed under light microscope. We evaluated IVF parameter by number of pronuclear formation: penetrated zygote (more than 2 pronuclear), monospermic fertilization (monospermy; with 2 pronuclear), and IVF efficiency (rate of monospermic zygotes to the number of penetrated oocytes).
After fertilization, spermatozoa surrounding zygotes were completely removed by gentle pipetting. Twenty fertilized zygotes were transferred to 100 µL drop of porcine zygote medium-3 (PZM-3) containing 0.3% (w/v) BSA (Sigma-Aldrich) and were cultured at 38.5° in 5% CO2 for 48 h. Then, 50 embryos were transferred to 650 µL of PZM-3 in 4-well culture plate and subsequently cultured for 120 h. The cleavage rate and blastocyst formation were evaluated at 48 and 168 h post-insemination, respectively.
Blastocysts at 7 day after insemination were used to measure cell number in blastocyst. Embryos at blastocyst stage were fixed in 4% (v/v) formaldehyde solution at RT for 3 min and were stained by 1 μg/mLbisbenzimide H 33342 trihydrochloride (Hoechst 33342; Sigma-Aldrich) at RT for 30 min in dark room. Stained nuclear in blastocysts were observed under fluorescent microscope using UV filter (400 nm).
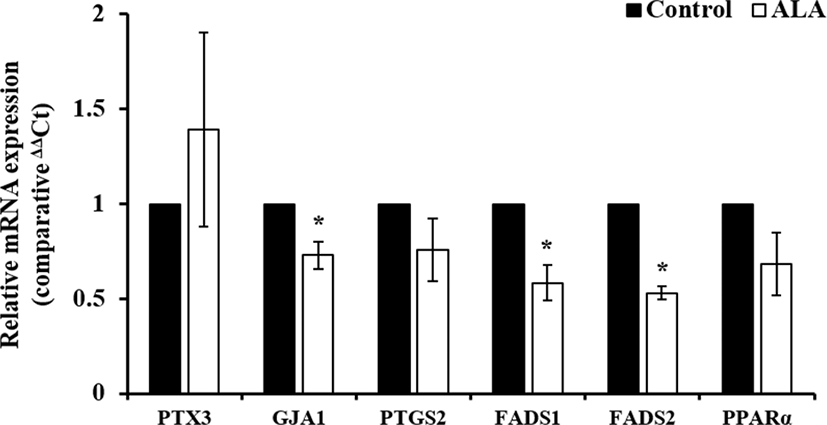
Total RNA extraction from cumulus cells, cultured with ALA for 44 h, was performed using trizol reagent (Takara, Japan) and cDNA was synthesized using Prime Script 1st strand cDNA Synthesis Kit (Takara) according to the manual. In brief, 1 µg of total RNA were incubated with 5 µM Oligo dT primer and 1 mM dNTP mixture at 65° for 5 min, and it was immediately cooled on ice after incubation. Then, RNA was mixed with 1 units/µl RNase inhibitor and 10 unit/µL RTase and mixture was reacted to synthesize cDNA at 42° for 1 h. Synthesized cDNA was quantified using NanoDrop 2000 (Thermo Scientific Nanodrop, Wilmington, DE, USA) and was diluted concentration at 50 ng/µL for real time PCR. All of primers for FAs metabolism- (delta-6 desaturase, FADS1; delta-5 desaturase, FADS2, peroxisome proliferator-activated receptor-alpha, PPARα) and cumulus expansion-related genes (pentraxin, PTX3; gap junction protein 1, GJA1; prostaglandin-endoperoxide synthase 2, PTGS2) was designed using Primer3 on-line software (Table 1). Thunderbird SYBR qPCR mix (Toyobo, Japan) was used for real-time PCR and cDNA amplification was performed following conditions: denaturation; 95° for 15 sec, annealing; 60° for 30 sec, extension; 72° for 30 sec. Relative expression level of genes were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and analyze by 2-∆∆Ct method.
FADS1, delta-6 desaturase; FADS2, delta-5desaturase; PPARa, peroxisome proliferator-activated receptor-alpha; PTX3, pentraxin; GJA1, gap junction protein alpha 1; PTGS2, prostaglandin-endoperoxide synthase 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase
-
Experiment 1: Effect of ALA during IVM on cell expansion, nuclear maturation, and gene expression of cumulus cells and oocytes
To evaluate effect of different concentration of ALA on cumulus cell expansion of COCs, porcine COCs were incubated in IVM medium with different concentration of ALA (0, 25, 50, and 100 µM) during first 22 h of IVM and cumulus cell expansion was measured using Image J. Based on result in cumulus cell expansion, nuclear maturation of oocytes and gene expression in cumulus cells that was incubated with 50 µM ALA at 44 h after maturation.
-
Experiment 2: Change of fertility in porcine oocytes mature with ALA
To confirm changes of fertility of oocytes by ALA, oocytes were incubated with ALA (0, 25, 50, and 100 μM) and were ferilized for 18–20 h. Then, sperm penetration and pronuclear formation were evaluated by aceto-orcein stain method.
-
Experiment 3: Effect of ALA during IVM on developmental competence of porcine oocytes
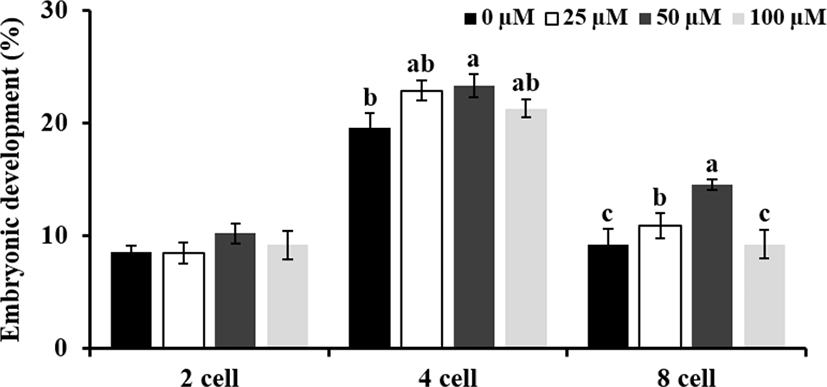
Experiment 3 was conducted to investigate whether treatment of ALA (0, 25, 50, and 100 μM) during maturation influence to development competence of oocytes. Therefore, cleavage rate and blastocyst formation were evaluated at 48 and 168 h after insemination, respectively. Especially, rates of embryos at 2, 4, and 8 cells stages were compare to investigate effect of ALA on early embryo development in pigs. As one of embryo quality parameters, total cell number in blastocyst at day 7 post-insemination was observed.
All numerical data representing each parameter were analyzed using the Statistical Analysis System Software (SAS version 9.4). Data are presented means±SEM, and comparisons among treatment groups were conducted by Duncan's multiple range tests (cumulus expansion, sperm penetration, and embryo development) and t-test (nuclear maturation and real time PCR) using a generalized linear model (GLM) in the SAS package. A value of p< 0.05 was considered to indicate a statistically significant difference.
RESULTS
-
Effect of ALA during IVM on cell expansion, nuclear maturation, and gene expression of cumulus cells and oocytes
Treatment of 50 μM ALA during first 22 h of IVM increased cumulus expansion compared to control and other treatment groups (Fig. 1; p<0.05) and ratio of meture oocytes at 44 h after IVM was higher in 50 μM ALA treatment group than control group (Fig. 2; p<0.05). Although cumulus expansion and nuclear maturation were enhanced, expression of GJA1, FADS1, and FADS2 mRNA in cumulus cells were reduced by treatment of 50 μM ALA (Fig. 3; p<0.05).
-
Change of fertilization in porcine oocytes mature with ALA
Change of IVF parameters by ALA during IVM was showed in Table 2. Sperm penetration was higher in 25 and 50 μM ALA groups than 100 μM ALA groups (p<0.05), however, it was not differ compared to control group. Both of 25 and 50 μM ALA enhanced monospermic fertilization (p<0.05). The IVF efficiency was increased in all of ALA treatment, however, there were no significant difference.
-
Effect of ALA during IVM on developmental competence of porcine oocytes
Embryonic developement at 48 h after IVF was increased by 25 and 50 μM ALA treatment compared to control group (Table 3; p<0.05), in particular, treatment of 50 μM ALA promoted early embryo develop to 4 and 8 cells stages (Fig. 4; p<0.05). Although both of 25 and 50 μM ALA treatment enhanced early embryo development, blastocyst formation and number of cells in blastocysts did not differ in 25 and 50 μM ALA groups compared to control group (Table 3).
DISCUSSION
Although IVP system of porcine embryos was improved by numerous reseaches, because of damage from in vitro environments, developmental competence of in vitro mature oocytes is still lower than in vivo mature oocytes (Guerin et al., 2001). Numerous reseaches have been tried to improve quality of IVM oocytes by supplementation of substances. FAs is one of energy source and it is contained in cytoplasm of porcine and bovine oocytes (McKeegan and Sturmey, 2011). Recently, many studies had reported that dietary supplement of PUFAs in cattle could enhanced reproductive performance (Wathes et al., 2007) and addition of oleic acid in maturation medium supported the development of oocyte via inhibition harmful effects of saturated FAs (Wang and Tsujii, 1999; Leroy et al., 2003; Aardema et al., 2011; Yenuganti et al., 2016; Fayezi et al., 2018).
This study was conducted to confirm effect of ALA during IVM on change of fertilization capacity and developmental competence by promoting cumulus expansion. Our fidings showed that cumulus expansion and nuclear maturation were increased by treatment of 50 μM ALA, whereas FAs metabolism- and cumulus expansion-related genes were down-regulated. Both of monospermic fertilization and early embryo development were also enhanced by 50 μM ALA, however, blastocyst formation was not influenced.
Cumulus cells play a very important role in delivering a variety of factors to the oocyte via gap junction communication (Gilula et al., 1978; Coskun et al., 1994). These transport of substances including metabolites and low size molecules by gap junction is closely associated with oocytes maturation. In present study, cumulus expansion and nuclear maturation were significantly increased by 50 μM ALA at 22 h and 44 h after maturation, respectively. Interaction between oocytes and cumulus cells was mediated by gap junction and it induced both of cumulus expansion and oocyte maturation. Especially, cysteine and glutamine that were transported by gap junction from cumulus cells into oocytes were synthesized to GSH in oocytes and synthesis of GSH is important indicator of cytoplasmic maturation (Mori et al., 2000). Lee et al. (2016a) reported that supplementation of ALA increased intracellular GSH level in porcine oocytes after IVM. Based on these research, we hypothesize that treatment of ALA promotes cumulus expansion and oocyte maturation via activation of gap junction at 22 h after maturation. Therefore, GSH synthesis in oocytes during maturation will be measured in further study.
FAs of metabolism and β-oxidation are closely associated with energy production, prostaglandins synthesis, imflammatory response, and other cellular processes (Foyer et al., 2003). As a precursor, ALA is synthesized to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) by FADS1, FADS2, and PPARα. In particular, PPARα regulates lipid metabolism, FAs transport, FAs binding and activation, and mitochondrial FAs β-oxidation, thereby it promotes the absorption, utilization and catabolism of FAs (Dunning et al., 2014; Lee et al., 2016b). We expected that addition of ALA during maturation period could induce expression of FAs metabolism-related genes. However, the expression of FADS1 and FADS2 mRNA was significantly reduced by 50 µM ALA treatment and PPARα mRNA was slightly decreased in ALA-treated cumulus cells at 44 h after maturation. Energy balance during maturation is crucial to nuclear and cytoplasmic maturation, and subsequent development in oocytes. However, excessive amount of FAs may be caused lipid peroxidation that is one of cause of reduced embryo quality (Lequarré et al., 2001; Reis et al., 2003). Therefore, we hypothesize that decreasing of FAs metabolism-related genes in ALA-treated cumulus cells could prevent excessive energy production and lipid peroxidation by ALA treatment. To investigate our hypothesis, expression of FAs metabolism-related genes, oxidative stress status, and lipid peroxidation according to culture time during maturation will be measured in further study.
Cumulus expansion of COCs is one of crucial parameter for evaluation of oocyte maturation and it is closely involved in function of gap junction. Various nutrients, metabolites, and substrates are transported through gap junction between cumulus cells and oocytes for sucessful maturation (Li et al., 2015). And several genes including PTX3, GJA1, and PTGS2 are involved with cumulus expansion and function of gap junction in mammalian oocytes (Zhang et al., 2005; Jia et al., 2014; Li et al., 2015). In present study, although treatment of 50 µM ALA enhanced cumulus expansion at 22 h after maturation, GJA1 mRNA in cumulus cells was downregulated at 44 h after maturation. And PTGS2 mRNA was slightly decreased, however, there were not significant difference. Reduction of GJA1 mRNA by ALA was similar with results of FA metabolism-related genes. Jia et al. (2014) reported that trans-10, cis-12 conjugated linoleic acid (t19c12 CLA) promoted protein expression of cyclo-oxygenase 2 (COX2; also known as PTGS2) in porcine oocytes for 22 h after maturation, whereas it was not differed at 44 h. These findings lead to predict that ALA treatment during first 22 h of maturation would promote FAs metabolism and gap junction for transport and production of energy, however, these are supressed to maintain energy balance during 22 h after first maturarion period. Therefore, our futher plan include invastigation of cumulus expansion-related genes and cAMP levels during first 22 h in IVM.
Formation of the zygote and embryo development after fertilization are considered as marker of the developmental ability in oocytes (Fayezi et al., 2018). During the maturation of the oocyte, incomplete cytoplasmic maturation results in polyspermy and lower development of embryos in vitro (Wang et al., 1997). In present study, monospermic fertilization and early embryonic development (developed to 4 and 8 cell) were increased by treatment of 25 and 50 μM ALA during IVM. As a marker of cellular maturation, GSH is increased in oocytes during maturation (DeMatos et al., 2000) and is involved with male pronuclei formation in oocytes (Mori et al., 2000). In our previous study, reduced GSH level in oocytes by H2O2 was recovered in 50 μM ALA and it suggested that ALA treatment could promote GSH synthesis in oocytes (Lee et al., 2017b). Furthermore, FAs are regarded as potential metabolic substrates in oocytes and early-stage embryos and are required as an energy source for the metabolism of embryos in pre-implantation of cows and pigs (Sturmey et al., 2009). Therefore, we expected that the addition of ALA could improve fertilization parameters and early development by increasing of GSH synthesis and energy production during maturation.
In conclusion, our findings show that 50 μM ALA during maturation enhanced cumulus expansion, nuclear maturation, monospermic fertilization, and early embryonic development, however, quality and formation of blastocyst were not influenced. Interestingly, FA metabolism- and cumulus expansion-related genes were reduced by ALA at 44 h after maturation. These results suggest that benefical effect and metabolism of ALA are occured during first 22 h of maturation and it might be closely assodicated with cytoplasmic maturation and gap junctional communication between oocytes and cumulus cells. Therefore, investigation of FA metabolism and energy production in oocytes during early maturation stage is needed.

