INTRODUCTION
Oocyte maturation process in teleosts is under the control or feedback of sex steroids, synthesized from the follicle cell, and they are regulated by the hypothalamus-pituitary –gonad axis (Nagahama & Yamashita, 2008; Planas & Swanson, 2008). In female fish, it is well known that estradiol-17β (E2) is the major sex steroid, and transported to liver during vitellogenesis, the period of vitellogenin production in liver. Subsequently, progestins function as such as maturation inducing steroid (MIS), 17α,20β-dihydroxy-4-pregnen-3-one (17α20βP) and/or 17α,20β,21-trihydroxy-4-pregnen-3-one (17α20β21P) induce final oocyte maturation, i.e., germinal vesicle breakdown (GVBD) and ovulation (Patiño & Sullivan, 2002; Baek, 2008; Nagahama & Yamashita, 2008). Although the actions and role of these sex steroids had been studied in many fish species, the profiling of various sex steroids in fish reproduction is still insufficient because of not only diverse fish species but their diverse reproductive characteristics (Frisch A, 2004; Tokarz et al., 2015).
The red-spotted grouper, Epinephelus akaara is a marine multiple spawner and protogynous, as a Serranid fish distributed mainly in Korea, southern Japan, southern China and East China Sea (Craig et al., 2011; Lee et al., 2020). This species is economically important in aquaculture industry and it is interested in artificial control of its reproduction such as induction of maturation and masculinization (Li et al., 2006; Lee et al., 2014; Oh et al., 2018; Lee et al., 2020). However, there is little information about sex steroid synthesis related to oocyte maturation of this species. In the present study, we investigated the in vitro profiling of sex steroid metabolites from ovarian follicles of red spotted grouper.
MATERIALS AND METHODS
Authentic steroids were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Steraloids (Wilton, NH, USA). Mother solutions (mg/mL) were prepared by dissolving the authentic steroids in pure ethanol. These were further diluted in incubation media. The ethanol concentration in the incubation medium was maintained at less than 0.1%. Radioactive [3H]-17α-hydroxyprogesterone ([3H]-17αP) was obtained from Amersham Life Science (London, UK).
The experimental fish were reared and adjusted to 14L:10D and 25°C in recirculating aquaria (500 L) from Marine Science Institute, Jeju National University, Korea. Oocytes were obtained by cannulation after anesthetization of eight mature females. The cannulated oocytes were put into cold balanced salt solution (BSS; 132.96 mM NaCl, 3.09 mM KCl, 0.28 mM MgSO4․7H2O, 0.98 mM MgCl2․6H2O, 3.40 mM CaCl2․6H2O, 3.65 mM HEPES). Oocytes were separated into groups with the largest diameter using fine forceps from each mature fish. Oocytes with average diameters of 350, 400, 420, 440, 480, and 500 μm were used for the in vitro incubation. Thirty follicle-enclosed oocytes were incubated in each well of triplicates.
We incubated isolated oocytes in 1 mL of Leibovitz L15 medium (Gibco, Grand Island, NY, USA) with 55 kBq [3H]-17αP a radiolabeled precursor for separation of metabolites. The pH and osmolality of the media were adjusted to 7.7 and 360 mOsm, respectively. The plates were incubated for 24 h at 18°C.
Some ovarian fragment from each fish were fixed, washed, dehydrated and paraffin-embedded. Sections of 4–5 μm were HE stained and observed through a light microscope.
At the end of the incubation, steroids extraction, separation and identification of metabolites were followed by our previous studies (Hwang et al., 2012; Baek et al., 2013; Lee & Baek, 2015). Steroid metabolites produced from [3H]-17αP were compared with the values were expressed as photo-stimulated luminescence (PSL) – background (BG) per mm2 of each fraction area from autoradiography.
RESULTS
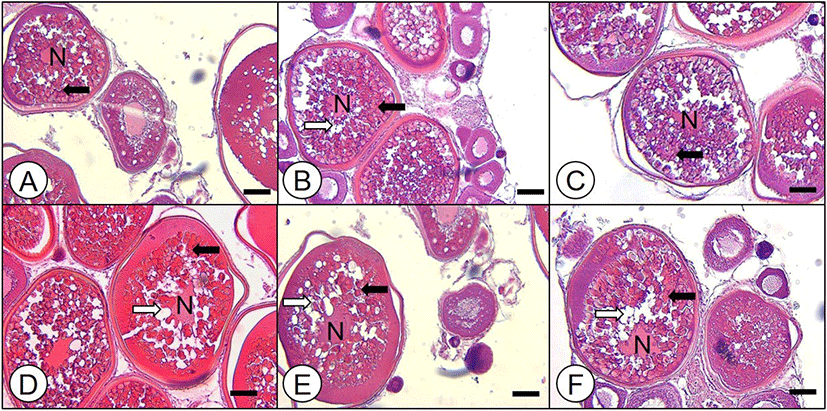
In histological observation of each diameter of oocytes, oocytes of 350–480 μm diameter were vitellogenic stage; yolk granules were spread throughout the cytoplasm and oil droplets (Od) were accumulated near the nucleus (N) as oocyte diameter increased (Fig. 1A–E). In particular, nucleus was observed in the middle of cytoplasm in these size of oocytes. In the oocytes of 500 μm diameter, migration of N was observed and many Ods were observed near migrating N (Fig. 1F).
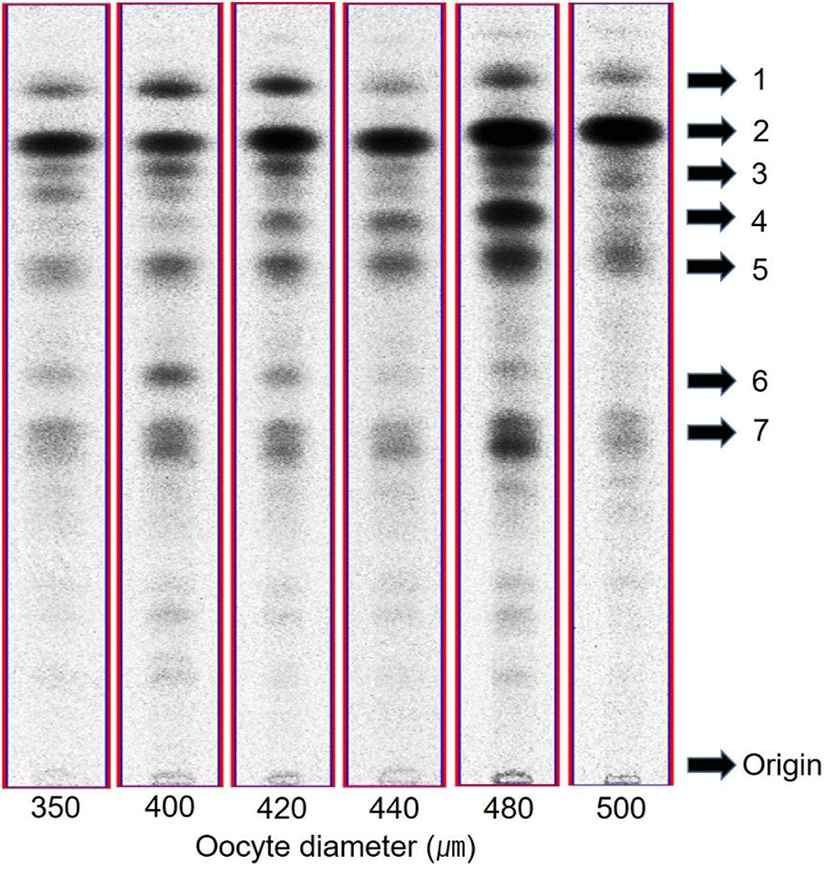
After incubation of oocytes with [3H]-17αP, 7 major fractions appeared on thin layer chromatography (TLC). Fraction 1, 2, 4, 5, and 6 were as the same retention factor (RF) of standard estrone (E1), androstenedione (A4), 17αP, T, and 17α20βP, respectively (Fig. 2). Fraction 3 and 7 were overlapped with E2+unknown metabolite and 17α,20α-dihydroxy-4-pregnen-3-one (17α20αP) +unknown metabolite, respectively. Among these 7 metabolites, fraction 1, 2, and 5 were identified as E1, A4, and T through further analysis with high performance liquid chromatography and GC/MS (Fig. 3). The other metabolites were not identified due to their low measured activities.
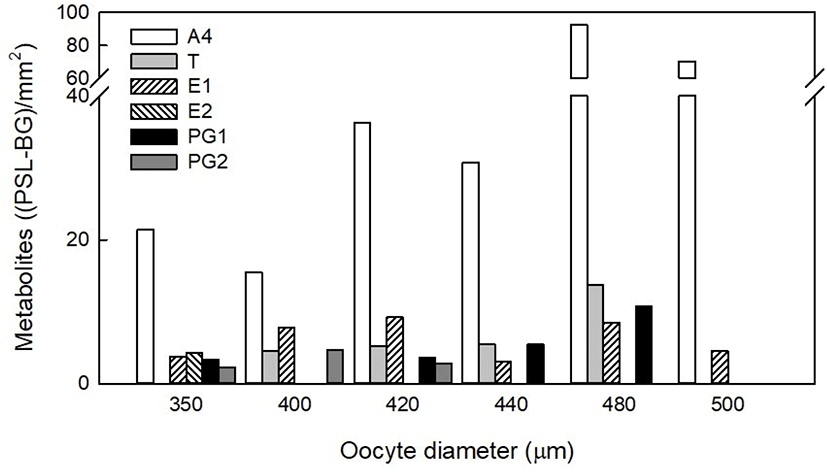
We compared each metabolite with PSL value from TLC autoradiography followed each oocyte diameter for steroid metabolites profiling during oocyte maturation (Fig. 4). The A4 metabolite was dominant [15.47–92.41 (PSL-BG)/mm2] in all size of oocytes and it was the highest in 480 μm diameter oocytes. T metabolite increased gradually [4.55–13.76 (PSL-BG)/mm2] as oocyte diameter increased although it was not detected at 350 and 500 μm diameter oocytes in the autoradiography. E2 overlapped with unknown metabolite was detected only in 350 μm diameter oocytes [4.27 (PSL-BG)/mm2]. E1 metabolite [3.08–9.24 (PSL-BG)/mm2] was detected in all size of oocytes and it was the highest in 420 μm diameter oocytes. 17α20βP metabolite was detected in 350–420 μm diameter oocytes [2.27–4.64 (PSL-BG)/mm2]. 17α20αP overlapped with unknown metabolite was detected 350, 420–480 μm diameter oocytes [3.37–10.82 (PSL-BG)/mm2] with the highest in 480 μm diameter oocytes. 17α20αP or 17α20βP metabolite was not detected in 500 μm diameter oocytes.
DISCUSSION
In general, E2, the principle estrogen is dominant in the early stage of maturation, i.e. oocyte growing and MIS such as 17α20βP or 17α20β21P dominant in the late stage of maturation, i.e. GVBD and ovulation (Tokarz et al., 2015). In histological observation of oocytes, 350–480 μm diameter oocytes were vitellogenic stage and 500 μm diameter oocytes were final maturation stage with migration of germinal vesicle (GV). We consider the 480 μm diameter oocytes would be fully vitellogenic stage; GV was still in the middle of cytoplasm and prior to GVM stage.
We identified the major steroid metabolites and investigate their profiling from vitellognic to germinal vesicle migration oocytes of red spotted grouper. The major metabolites synthesized from the oocytes were A4, E1, T, E2 and two progestins. Among these metabolites, A4, E1, and T were identified through GC/MS, but the others were not identified due to their low activities. Although we could not identify 2 progestins, progestin 1 and 2 co-migrated with 17α20αP and 17α20βP in TLC system. To date, MIS of groupers had not been identified yet although 17α20βP is well-known MIS in many fish species and was measured from plasma of Epinephelus morio (Johnson et al., 1998; Shein et al., 2004; Nagahama & Yamashita, 2008). Interestingly, progestin 1 metabolite, suspected 17α20αP increased gradually in 420–480 μm diameter oocytes. Previous studies reported that 17α20αP may act as a MIS of flatfish, Limanda limanda, Verasper variegates and gobiid fish, Chasmichthys dolichognathus (Canario & Scott, 1990; Baek, 2001, 2008). In this regard, 17α20αP metabolite would be related to maturation process with its increase until fully vitellogenic stage and then decrease at GVM stage although its role is not clear. Further study that identification of MIS and it’s maturation inducing capacity should be conducted.
Among the metabolites, A4 was predominant in all size of oocytes even in the GVM stage (500 μm oocyte diameter). In our previous study, A4 was also produced dominantly in vitellogenic oocytes from red lip mullet, Chelon haematocheilus, blacktip grouper, Epinephelus fasciatus, and blackfin flounder, Glyptocephalus stelleri (Baek et al., 2011; Hwang et al., 2012; Lee & Baek, 2015). A few studies reported that A4 is related to sex change in protogynous hermaphrodite, Monopterus albus and Sparisoma viride (Yeung & Chan, 1987; Cardwell & Liley, 1991; Yeung et al., 1993). Moreover, Montero et al. (1995) reported that androgen could act as a substrate for estrogen production in vitellogenesis of European eel, Anguilla anguilla. In the present study, we suspect that A4 may regulate oocyte maturation process rather than sex change of red spotted grouper since there was no testicular tissue in gonad and it’s the highest value with progestin production at 480 μm diameter oocytes, the transitional stage into final maturation.
We demonstrated that E1 metabolite was also detected in all size of oocytes and its detected value was higher than that of E2 metabolite. In a majority of fishes, it is well known that E2 is converted from T by aromatase (Fukada et al., 1996; Chang et al., 1997; Aggarwal et al., 2014). However, E1 may have a minor vitellogenic role in some species (Specker & Sullivan, 1994; Routledge et al., 1998) and act as a precursor of E2 synthesis (Kazeto et al., 2000; Ohta et al., 2001; Ohta et al., 2002; Mindnich et al., 2004; Zhou et al., 2005). Our results also suggest that E1 may regulate vitellogenesis with E2 and also has a certain role in oocyte maturation process despite insufficient data. Future study such as exogenous E1 treatment on oocyte maturation would provide more definite information about steroidogenic profiling. In conclusion, the present study provides in vitro steroid metabolites profiling that identification of major metabolites and its’s changes during oocytes maturation of red spotted grouper. We also suggest that 17α20βP and/or 17α20αP may act as a MIS and the potential role of E1 in maturation process.

